当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Arc silence aggravates traumatic neuronal injury via mGluR1-mediated ER stress and necroptosis.
Cell Death & Disease ( IF 9 ) Pub Date : 2020-01-02 , DOI: 10.1038/s41419-019-2198-5 Tao Chen 1, 2 , Jie Zhu 2 , Yu-Hai Wang 2 , Chun-Hua Hang 1
Cell Death & Disease ( IF 9 ) Pub Date : 2020-01-02 , DOI: 10.1038/s41419-019-2198-5 Tao Chen 1, 2 , Jie Zhu 2 , Yu-Hai Wang 2 , Chun-Hua Hang 1
Affiliation
|
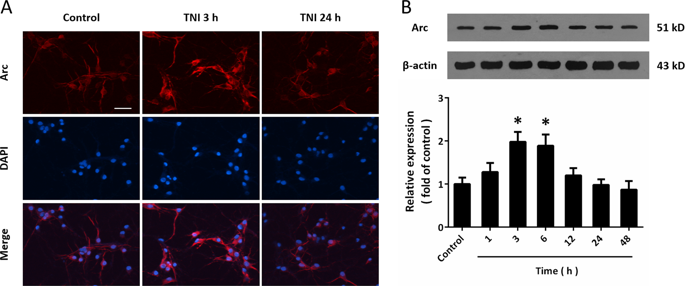
Delayed neuronal death is associated with neurological deficits and mortality after traumatic brain injury (TBI), where post-synaptic density (PSD) proteins are thought to play key roles. The immediate-early gene (IEG) coded protein Arc is a brain-specific PSD protein that controls synaptic plasticity and learning behaviors. In this study, we investigated the expression and biological function of Arc in neuronal death after TBI in an in vitro model mimicked by traumatic neuronal injury (TNI) in cortical neurons. TNI caused a temporal increase of Arc expression at 3 and 6 h. Knockdown of Arc expression using small interfering RNA (Si-Arc-3) promoted TNI-induced cytotoxicity and apoptosis. The results of western blot showed that Si-Arc-3 transfection further enhanced the activation of endoplasmic reticulum (ER) stress-associated factors, including glucose-regulated protein 78 (GRP78), C/EBP homologous protein (CHOP) and caspase-12 after TNI. In addition, knockdown of Arc significantly increased expression of (receptor-interacting protein kinase 1) RIP1 and the number of necroptotic cells, which were apparently prevented by necrostatin-1 (Nec-1). The results of immunostaining and western blot showed that knockdown of Arc activated the metabotropic glutamate receptor 1 (mGluR1) and intracellular Ca2+ release in neurons. Mechanistically, the Si-Arc-3-induced activation of ER stress-associated factors, RIP1 expression, apoptosis, and necroptosis were partially reversed by the mGluR1 antagonist AIDA. In summary, our data suggest that silence of Arc expression aggravates neuronal death after TNI by promoting apoptosis and necroptosis. These data support for the first time that Arc may represent a novel candidate for therapies against TBI.
中文翻译:

弧形沉默通过mGluR1介导的内质网应激和坏死加剧了神经元的损伤。
延迟性神经元死亡与创伤性脑损伤(TBI)后的神经功能缺损和死亡率相关,其中突触后密度(PSD)蛋白被认为起关键作用。立即早期基因(IEG)编码的蛋白Arc是一种大脑特异性的PSD蛋白,它控制突触的可塑性和学习行为。在这项研究中,我们调查了皮质神经元的创伤性神经元损伤(TNI)模拟的体外模型中,TBI后神经元死亡中Arc的表达和生物学功能。TNI导致3和6小时时Arc表达随时间增加。使用小干扰RNA(Si-Arc-3)抑制Arc表达促进了TNI诱导的细胞毒性和细胞凋亡。Western blot结果显示,Si-Arc-3转染进一步增强了内质网(ER)应激相关因子的激活,包括TNI后的葡萄糖调节蛋白78(GRP78),C / EBP同源蛋白(CHOP)和caspase-12。此外,Arc的敲低显着增加了(与受体相互作用的蛋白激酶1)RIP1的表达和坏死性细胞的数量,这显然被necrostatin-1(Nec-1)阻止了。免疫染色和蛋白质印迹的结果表明,敲除Arc激活了代谢型谷氨酸受体1(mGluR1)和神经元中的细胞内Ca2 +释放。从机制上讲,mGluR1拮抗剂AIDA可以部分逆转Si-Arc-3-诱导的ER应激相关因子,RIP1表达,凋亡和坏死性坏死。总之,我们的数据表明,Arc表达的沉默通过促进细胞凋亡和坏死病加重了TNI后的神经元死亡。
更新日期:2020-01-09
中文翻译:

弧形沉默通过mGluR1介导的内质网应激和坏死加剧了神经元的损伤。
延迟性神经元死亡与创伤性脑损伤(TBI)后的神经功能缺损和死亡率相关,其中突触后密度(PSD)蛋白被认为起关键作用。立即早期基因(IEG)编码的蛋白Arc是一种大脑特异性的PSD蛋白,它控制突触的可塑性和学习行为。在这项研究中,我们调查了皮质神经元的创伤性神经元损伤(TNI)模拟的体外模型中,TBI后神经元死亡中Arc的表达和生物学功能。TNI导致3和6小时时Arc表达随时间增加。使用小干扰RNA(Si-Arc-3)抑制Arc表达促进了TNI诱导的细胞毒性和细胞凋亡。Western blot结果显示,Si-Arc-3转染进一步增强了内质网(ER)应激相关因子的激活,包括TNI后的葡萄糖调节蛋白78(GRP78),C / EBP同源蛋白(CHOP)和caspase-12。此外,Arc的敲低显着增加了(与受体相互作用的蛋白激酶1)RIP1的表达和坏死性细胞的数量,这显然被necrostatin-1(Nec-1)阻止了。免疫染色和蛋白质印迹的结果表明,敲除Arc激活了代谢型谷氨酸受体1(mGluR1)和神经元中的细胞内Ca2 +释放。从机制上讲,mGluR1拮抗剂AIDA可以部分逆转Si-Arc-3-诱导的ER应激相关因子,RIP1表达,凋亡和坏死性坏死。总之,我们的数据表明,Arc表达的沉默通过促进细胞凋亡和坏死病加重了TNI后的神经元死亡。



























 京公网安备 11010802027423号
京公网安备 11010802027423号