当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spontaneous Glycan Reattachment Following N-Glycanase Treatment of Influenza and HIV Vaccine Antigens.
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.jproteome.9b00620 Celina L Keating 1 , Eric Kuhn 2 , Julia Bals 1 , Alexandra R Cocco 2 , Ashraf S Yousif 1 , Colette Matysiak 1 , Maya Sangesland 1 , Larance Ronsard 1 , Matthew Smoot 1 , Thalia Bracamonte Moreno 1 , Vintus Okonkwo 1 , Ian Setliff 3, 4 , Ivelin Georgiev 3, 4, 5, 6 , Alejandro B Balazs 1 , Steven A Carr 2 , Daniel Lingwood 1
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.jproteome.9b00620 Celina L Keating 1 , Eric Kuhn 2 , Julia Bals 1 , Alexandra R Cocco 2 , Ashraf S Yousif 1 , Colette Matysiak 1 , Maya Sangesland 1 , Larance Ronsard 1 , Matthew Smoot 1 , Thalia Bracamonte Moreno 1 , Vintus Okonkwo 1 , Ian Setliff 3, 4 , Ivelin Georgiev 3, 4, 5, 6 , Alejandro B Balazs 1 , Steven A Carr 2 , Daniel Lingwood 1
Affiliation
|
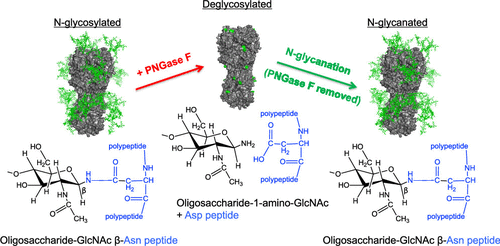
In cells, asparagine/N-linked glycans are added to glycoproteins cotranslationally, in an attachment process that supports proper folding of the nascent polypeptide. We found that following pruning of N-glycan by the amidase PNGase F, the principal influenza vaccine antigen and major viral spike protein hemagglutinin (HA) spontaneously reattached N-glycan to its de-N-glycosylated positions when the amidase was removed from solution. This reaction, which we term N-glycanation, was confirmed by site-specific analysis of HA glycoforms by mass spectrometry prior to PNGase F exposure, during exposure to PNGase F, and after amidase removal. Iterative rounds of de-N-glycosylation followed by N-glycanation could be repeated at least three times and were observed for other viral glycoproteins/vaccine antigens, including the envelope glycoprotein (Env) from HIV. Covalent N-glycan reattachment was nonenzymatic as it occurred in the presence of metal ions that inhibit PNGase F activity. Rather, N-glycanation relied on a noncovalent assembly between protein and glycan, formed in the presence of the amidase, where linearization of the glycoprotein prevented this retention and subsequent N-glycanation. This reaction suggests that under certain experimental conditions, some glycoproteins can organize self-glycan addition, highlighting a remarkable self-assembly principle that may prove useful for re-engineering therapeutic glycoproteins such as influenza HA or HIV Env, where glycan sequence and structure can markedly affect bioactivity and vaccine efficacy.
中文翻译:

N-糖基化酶处理流感和HIV疫苗抗原后,自发糖基重新附着。
在细胞中,天冬酰胺/ N-连接的聚糖以支持新生多肽正确折叠的附着过程共翻译地添加到糖蛋白中。我们发现,在酰胺酶PNGase F修剪N-聚糖后,当从溶液中去除酰胺酶时,主要的流感疫苗抗原和主要的病毒杀伤蛋白血凝素(HA)自发地将N-聚糖重新连接至其去N-糖基化位置。在PNGase F暴露之前,PNGase F暴露期间和酰胺酶去除之后,通过质谱对HA糖型进行位点特异性分析,证实了该反应(我们称为N-糖基化)。去N-糖基化再进行N-糖基化的迭代轮可以重复至少3次,并观察到其他病毒糖蛋白/疫苗抗原,包括来自HIV的包膜糖蛋白(Env)。共价N-聚糖重新连接是非酶促的,因为它在抑制PNGase F活性的金属离子存在下发生。而是,N-糖基化依赖于在酰胺酶存在下形成的蛋白质和聚糖之间的非共价组装,其中糖蛋白的线性化阻止了这种保留和随后的N-糖基化。该反应表明,在某些实验条件下,某些糖蛋白可以组织自糖基的添加,突出了非凡的自组装原理,这可能被证明可用于重组治疗性糖蛋白,例如流感病毒HA或HIV Env,其中糖基序列和结构可以明显地影响生物活性和疫苗效力。共价N-聚糖重新连接是非酶促的,因为它在抑制PNGase F活性的金属离子存在下发生。而是,N-糖基化依赖于在酰胺酶存在下形成的蛋白质和聚糖之间的非共价组装,其中糖蛋白的线性化阻止了这种保留和随后的N-糖基化。该反应表明,在某些实验条件下,某些糖蛋白可以组织自糖基的添加,突出了非凡的自组装原理,这可能被证明可用于重组治疗性糖蛋白,例如流感病毒HA或HIV Env,其中糖基序列和结构可以明显地影响生物活性和疫苗效力。共价N-聚糖重新连接是非酶促的,因为它在抑制PNGase F活性的金属离子存在下发生。而是,N-糖基化依赖于在酰胺酶存在下形成的蛋白质和聚糖之间的非共价组装,其中糖蛋白的线性化阻止了这种保留和随后的N-糖基化。该反应表明,在某些实验条件下,某些糖蛋白可以组织自糖基的添加,突出了非凡的自组装原理,这可能被证明可用于重组治疗性糖蛋白,例如流感病毒HA或HIV Env,其中糖基序列和结构可以明显地影响生物活性和疫苗效力。其中糖蛋白的线性化阻止了这种保留和随后的N-糖基化。该反应表明,在某些实验条件下,某些糖蛋白可以组织自糖基的添加,突出了非凡的自组装原理,这可能被证明可用于重组治疗性糖蛋白,例如流感病毒HA或HIV Env,其中糖基序列和结构可以明显地影响生物活性和疫苗效力。其中糖蛋白的线性化阻止了这种保留和随后的N-糖基化。该反应表明,在某些实验条件下,某些糖蛋白可以组织自糖基的添加,突出了非凡的自组装原理,这可能被证明可用于重组治疗性糖蛋白,例如流感病毒HA或HIV Env,其中糖基序列和结构可以明显地影响生物活性和疫苗效力。
更新日期:2020-01-26
中文翻译:

N-糖基化酶处理流感和HIV疫苗抗原后,自发糖基重新附着。
在细胞中,天冬酰胺/ N-连接的聚糖以支持新生多肽正确折叠的附着过程共翻译地添加到糖蛋白中。我们发现,在酰胺酶PNGase F修剪N-聚糖后,当从溶液中去除酰胺酶时,主要的流感疫苗抗原和主要的病毒杀伤蛋白血凝素(HA)自发地将N-聚糖重新连接至其去N-糖基化位置。在PNGase F暴露之前,PNGase F暴露期间和酰胺酶去除之后,通过质谱对HA糖型进行位点特异性分析,证实了该反应(我们称为N-糖基化)。去N-糖基化再进行N-糖基化的迭代轮可以重复至少3次,并观察到其他病毒糖蛋白/疫苗抗原,包括来自HIV的包膜糖蛋白(Env)。共价N-聚糖重新连接是非酶促的,因为它在抑制PNGase F活性的金属离子存在下发生。而是,N-糖基化依赖于在酰胺酶存在下形成的蛋白质和聚糖之间的非共价组装,其中糖蛋白的线性化阻止了这种保留和随后的N-糖基化。该反应表明,在某些实验条件下,某些糖蛋白可以组织自糖基的添加,突出了非凡的自组装原理,这可能被证明可用于重组治疗性糖蛋白,例如流感病毒HA或HIV Env,其中糖基序列和结构可以明显地影响生物活性和疫苗效力。共价N-聚糖重新连接是非酶促的,因为它在抑制PNGase F活性的金属离子存在下发生。而是,N-糖基化依赖于在酰胺酶存在下形成的蛋白质和聚糖之间的非共价组装,其中糖蛋白的线性化阻止了这种保留和随后的N-糖基化。该反应表明,在某些实验条件下,某些糖蛋白可以组织自糖基的添加,突出了非凡的自组装原理,这可能被证明可用于重组治疗性糖蛋白,例如流感病毒HA或HIV Env,其中糖基序列和结构可以明显地影响生物活性和疫苗效力。共价N-聚糖重新连接是非酶促的,因为它在抑制PNGase F活性的金属离子存在下发生。而是,N-糖基化依赖于在酰胺酶存在下形成的蛋白质和聚糖之间的非共价组装,其中糖蛋白的线性化阻止了这种保留和随后的N-糖基化。该反应表明,在某些实验条件下,某些糖蛋白可以组织自糖基的添加,突出了非凡的自组装原理,这可能被证明可用于重组治疗性糖蛋白,例如流感病毒HA或HIV Env,其中糖基序列和结构可以明显地影响生物活性和疫苗效力。其中糖蛋白的线性化阻止了这种保留和随后的N-糖基化。该反应表明,在某些实验条件下,某些糖蛋白可以组织自糖基的添加,突出了非凡的自组装原理,这可能被证明可用于重组治疗性糖蛋白,例如流感病毒HA或HIV Env,其中糖基序列和结构可以明显地影响生物活性和疫苗效力。其中糖蛋白的线性化阻止了这种保留和随后的N-糖基化。该反应表明,在某些实验条件下,某些糖蛋白可以组织自糖基的添加,突出了非凡的自组装原理,这可能被证明可用于重组治疗性糖蛋白,例如流感病毒HA或HIV Env,其中糖基序列和结构可以明显地影响生物活性和疫苗效力。

























 京公网安备 11010802027423号
京公网安备 11010802027423号