当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Performances and Mechanism of Methyl Orange and Congo Red Adsorbed on the Magnetic Ion-Exchange Resin
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jced.9b00951 Yunhan Jia 1 , Lei Ding 1, 2 , Peiyue Ren 1 , Meiying Zhong 1, 2 , Jiangya Ma 1, 2 , Xiaoran Fan 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jced.9b00951 Yunhan Jia 1 , Lei Ding 1, 2 , Peiyue Ren 1 , Meiying Zhong 1, 2 , Jiangya Ma 1, 2 , Xiaoran Fan 1
Affiliation
|
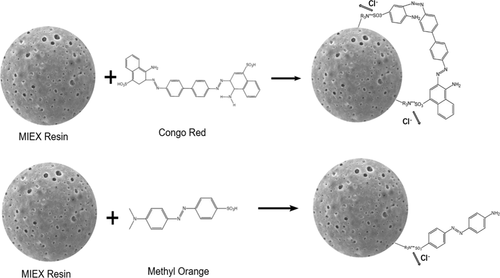
Effective removal of azo dyes is a stringent issue. In this study, the adsorption behaviors of methyl orange (MO) and congo red (CR) on the magnetic ion-exchange (MIEX) resin were investigated by batch experiments. Attaining adsorption equilibrium of MO and CR takes 90 and 150 min, respectively. The equilibrium adsorption capacity of MO (42.32 mg mL–1) on the MIEX resin is much larger than that of CR (30.57 mg mL–1). The kinetic processes of MO and CR adsorbed on the MIEX resin follows the Elovich model. At 288 K, the Langmuir model can simulate the equilibrium adsorption data of MO and CR. However, the equilibrium data can be well fitted by the Freundlich model at 293 and 303 K. The thermodynamic parameters show that the adsorption of MO and CR are both thermodynamically spontaneous, endothermic processes. The MIEX resin can well remove MO (80%) and CR (50%) at pH 5.0–9.0. Ion exchange dominates the mechanism of CR adsorbed on the MIEX resin. For MO, however, physical adsorption plays a role to some extent besides ion exchange. Sodium chloride (187 mg) is needed to regenerate per milliliter of spent the MIEX resin. After eight times adsorption–desorption cycles, The MIEX resin retains still efficient adsorption. As a summary, the MIEX resin can be considered as a promising adsorbent to remove azo dyes from water and wastewater due to its high adsorption capacity, short adsorption time, and simple regeneration.
中文翻译:

磁性离子交换树脂吸附甲基橙和刚果红的性能及机理
有效去除偶氮染料是一个严格的问题。通过分批实验研究了甲基橙(MO)和刚果红(CR)在磁性离子交换(MIEX)树脂上的吸附行为。达到MO和CR的吸附平衡分别需要90和150分钟。MO(42.32 mg mL –1)在MIEX树脂上的平衡吸附容量远远大于CR(30.57 mg mL –1)的平衡吸附容量。)。吸附在MIEX树脂上的MO和CR的动力学过程遵循Elovich模型。在288 K时,Langmuir模型可以模拟MO和CR的平衡吸附数据。但是,平衡数据可以通过293和303 K时的Freundlich模型很好地拟合。热力学参数表明MO和CR的吸附都是热力学自发的吸热过程。在pH 5.0-9.0的条件下,MIEX树脂可以很好地去除MO(80%)和CR(50%)。离子交换控制CR吸附在MIEX树脂上的机理。但是,对于MO,物理吸附在一定程度上起着离子交换的作用。每毫升废MIEX树脂需要再生氯化钠(187 mg)。经过八次吸附-解吸循环后,MIEX树脂仍保持有效吸附。总结一下,
更新日期:2020-01-09
中文翻译:

磁性离子交换树脂吸附甲基橙和刚果红的性能及机理
有效去除偶氮染料是一个严格的问题。通过分批实验研究了甲基橙(MO)和刚果红(CR)在磁性离子交换(MIEX)树脂上的吸附行为。达到MO和CR的吸附平衡分别需要90和150分钟。MO(42.32 mg mL –1)在MIEX树脂上的平衡吸附容量远远大于CR(30.57 mg mL –1)的平衡吸附容量。)。吸附在MIEX树脂上的MO和CR的动力学过程遵循Elovich模型。在288 K时,Langmuir模型可以模拟MO和CR的平衡吸附数据。但是,平衡数据可以通过293和303 K时的Freundlich模型很好地拟合。热力学参数表明MO和CR的吸附都是热力学自发的吸热过程。在pH 5.0-9.0的条件下,MIEX树脂可以很好地去除MO(80%)和CR(50%)。离子交换控制CR吸附在MIEX树脂上的机理。但是,对于MO,物理吸附在一定程度上起着离子交换的作用。每毫升废MIEX树脂需要再生氯化钠(187 mg)。经过八次吸附-解吸循环后,MIEX树脂仍保持有效吸附。总结一下,



























 京公网安备 11010802027423号
京公网安备 11010802027423号