当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly efficient asymmetric reduction of ketopantolactone to D-(−)-pantolactone by Escherichia coli cells expressing recombinant conjugated polyketone reductase and glucose dehydrogenase in a fed-batch biphasic reaction system
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2020/01/08 , DOI: 10.1039/c9re00385a Xiaolin Pei 1, 2, 3, 4, 5 , Jiapao Wang 1, 2, 3, 4 , Haoteng Zheng 1, 2, 3, 4 , Pengfei Cheng 3, 4, 5, 6, 7 , Yifeng Wu 1, 2, 3, 4 , Anming Wang 1, 2, 3, 4 , Weike Su 3, 4, 5, 6, 7
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2020/01/08 , DOI: 10.1039/c9re00385a Xiaolin Pei 1, 2, 3, 4, 5 , Jiapao Wang 1, 2, 3, 4 , Haoteng Zheng 1, 2, 3, 4 , Pengfei Cheng 3, 4, 5, 6, 7 , Yifeng Wu 1, 2, 3, 4 , Anming Wang 1, 2, 3, 4 , Weike Su 3, 4, 5, 6, 7
Affiliation
|
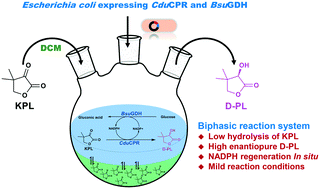
D-(−)-Pantolactone (D-PL) is the key chiral intermediate used to synthesize calcium D-pantothenate. In a previous study, a conjugated polyketone reductase (CduCPR) was discovered to efficiently catalyse ketopantolactone (KPL) to D-PL with a high stereoselectivity. Nevertheless, this method was limited due to the expensive coenzyme, nicotinamide adenine dinucleotide phosphate hydrate (NADPH), and the poor stability of substrate KPL in pure aqueous buffer. Here, to overcome these problems, we developed a whole-cell biotransformation process to produce D-PL in a biphasic reaction system. Recombinant CduCPR and glucose dehydrogenase (BsuGDH) were co-expressed in Escherichia coli to simultaneously achieve the synthesis of D-PL and the regeneration of NADPH. The biphasic reaction system contained 15% dichloromethane (v/v) which significantly inhibited the undesirable hydrolysis of KPL. Approximately, 2.8% of 100 mM KPL was spontaneously hydrolysed to ketopantoic acid in the biphasic system, which was lower than 54% of pure aqueous buffer. The biphasic reaction system was optimized and scaled up to prepare D-PL. In a fed-batch biphasic reaction, the substrate KPL was continuously fed into the reactor after a batch reaction. The D-PL concentration reached 0.77 mol L−1 in the reaction mixture at 7 h, and its enantiomeric excess was 99%. This study presented an alternative method to asymmetrically synthesize enantiopure D-PL for further industrial application.
中文翻译:

在补料分批双相反应系统中表达重组共轭聚酮还原酶和葡萄糖脱氢酶的大肠杆菌细胞将酮基内酯高效不对称还原为D-(-)-泛内酯
D -(-)-戊内酯(D -PL)是用于合成D-泛酸钙的关键手性中间体。在先前的研究中,发现共轭聚酮还原酶(Cdu CPR)可以高立体选择性将酮基内酯(KPL)有效地催化成D -PL。但是,由于昂贵的辅酶,烟酰胺腺嘌呤二核苷酸磷酸水合物(NADPH)以及底物KPL在纯水缓冲液中的稳定性差,因此该方法受到了限制。在这里,为了克服这些问题,我们开发了一种全细胞生物转化方法,以在双相反应系统中产生D -PL。重组Cdu CPR和葡萄糖脱氢酶(BsuGDH)在大肠杆菌中共表达,以同时实现D -PL的合成和NADPH的再生。双相反应系统包含15%的二氯甲烷(v / v),可显着抑制KPL的不良水解。在两相系统中,约有2.8%的100 mM KPL自发水解为酮戊酸,低于纯水性缓冲液的54%。优化了两相反应系统并按比例放大以制备D -PL。在分批补料的两相反应中,在分批反应后将底物KPL连续进料到反应器中。所述d -PL浓度达到0.77摩尔大号-1在反应混合物中在7小时时,其对映体过量为99%。这项研究提出了一种不对称合成对映体D -PL的替代方法,可用于进一步的工业应用。
更新日期:2020-03-03
中文翻译:

在补料分批双相反应系统中表达重组共轭聚酮还原酶和葡萄糖脱氢酶的大肠杆菌细胞将酮基内酯高效不对称还原为D-(-)-泛内酯
D -(-)-戊内酯(D -PL)是用于合成D-泛酸钙的关键手性中间体。在先前的研究中,发现共轭聚酮还原酶(Cdu CPR)可以高立体选择性将酮基内酯(KPL)有效地催化成D -PL。但是,由于昂贵的辅酶,烟酰胺腺嘌呤二核苷酸磷酸水合物(NADPH)以及底物KPL在纯水缓冲液中的稳定性差,因此该方法受到了限制。在这里,为了克服这些问题,我们开发了一种全细胞生物转化方法,以在双相反应系统中产生D -PL。重组Cdu CPR和葡萄糖脱氢酶(BsuGDH)在大肠杆菌中共表达,以同时实现D -PL的合成和NADPH的再生。双相反应系统包含15%的二氯甲烷(v / v),可显着抑制KPL的不良水解。在两相系统中,约有2.8%的100 mM KPL自发水解为酮戊酸,低于纯水性缓冲液的54%。优化了两相反应系统并按比例放大以制备D -PL。在分批补料的两相反应中,在分批反应后将底物KPL连续进料到反应器中。所述d -PL浓度达到0.77摩尔大号-1在反应混合物中在7小时时,其对映体过量为99%。这项研究提出了一种不对称合成对映体D -PL的替代方法,可用于进一步的工业应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号