当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CO2 Reduction: From Homogeneous to Heterogeneous Electrocatalysis.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.accounts.9b00496 Sheng Zhang 1 , Qun Fan 1 , Rong Xia 1 , Thomas J Meyer 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.accounts.9b00496 Sheng Zhang 1 , Qun Fan 1 , Rong Xia 1 , Thomas J Meyer 2
Affiliation
|
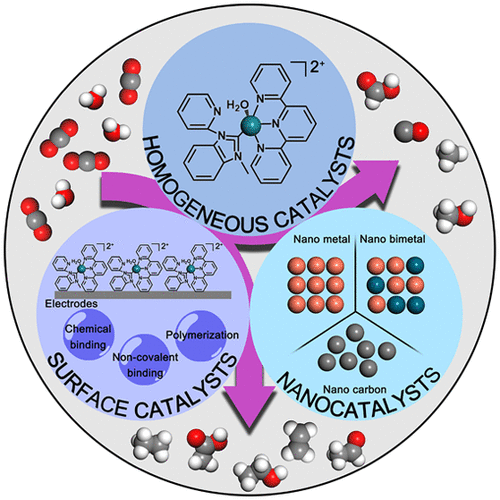
Due to increasing worldwide fossil fuel consumption, carbon dioxide levels have increased in the atmosphere with increasingly important impacts on the environment. Renewable and clean sources of energy have been proposed, including wind and solar, but they are intermittent and require efficient and scalable energy storage technologies. Electrochemical CO2 reduction reaction (CO2RR) provides a valuable approach in this area. It combines solar- or wind-generated electrical production with energy storage in the chemical bonds of carbon-based fuels. It can provide ways to integrate carbon capture, utilization, and storage in energy cycles while maintaining controlled levels of atmospheric CO2. Electrochemistry allows for the utilization of an electrical input to drive chemical reactions. Because CO2 is kinetically inert, highly active catalysts are required to decrease reaction barriers sufficiently so that reaction rates can be achieved that are sufficient for electrochemical CO2 reduction. Given the reaction barriers associated with multiple electron-proton reduction of CO2 to CO, formaldehyde (HC(O)H), formic acid, or formate (HC(O)OH, HC(O)O-), or more highly reduced forms of carbon, there is also a demand for high selectivity in catalysis. Catalysts that have been explored include homogeneous catalysts in solution, catalysts immobilized on surfaces, and heterogeneous catalysts. In homogeneous catalysis, reduction occurs following diffusion of the catalyst to an electrode where multiple proton coupled electron transfer reduction occurs. Useful catalysts in this area are typically transition-metal complexes with organic ligands and electron transfer properties that utilize combinations of metal and ligand redox levels. As a way to limit the amount of catalyst, in device-like configurations, catalysts are added to the surfaces of conductive substrates by surface binding, in polymeric films, or on carbon electrode surfaces with molecular structures and electronic configurations related to catalysts in solution. Immobilized, homogeneous catalysts can suffer from performance losses and even decomposition during long-term CO2 reduction cycles, but they are amenable to detailed mechanistic investigations. In parallel efforts, heterogeneous nanocatalysts have been explored in detail with the development of facile synthetic procedures that can offer highly active catalytic surface areas. Their high activity and stability have attracted a significant level of investigation, including possible exploitation for large-scale applications. However, translation of catalytic reactivity to the surface creates a new reactivity environment and complicates the elucidation of mechanistic details and identification of the active site in exploring reaction pathways. Here, the results of previous studies based on transition-metal complex catalysts for CO2 electroreduction are summarized. Early studies showed that transition-metal complexes of Ru, Ir, Rh, and Os, with well-defined structures, are all capable of catalyzing CO2 reduction to CO or formate. Derivatives of the complexes were surface attached to conducting electrodes by chemical bonding, noncovalent bonding, or polymerization. The concept of surface binding has also been extended to the preparation of surface area electrodes by the chemically controlled deposition of nanostructured catalysts such as nano tin, nano copper, and nano carbon, all of which have been shown to have high selectivities and activities toward CO2 reduction. In our presentation, we end this Account with recent advances and a perspective about the application of electrocatalysis in carbon dioxide reduction.
中文翻译:

减少二氧化碳:从均相到多相电催化。
由于世界范围内化石燃料消耗的增加,大气中二氧化碳的含量增加,对环境的影响日益重要。已经提出了可再生的清洁能源,包括风能和太阳能,但是它们是间歇性的,需要高效且可扩展的储能技术。电化学二氧化碳还原反应(CO2RR)在这一领域提供了一种有价值的方法。它结合了太阳能或风能发电与碳基燃料化学键中的能量存储。它可以提供在能量循环中整合碳捕获,利用和存储的方法,同时保持受控的大气CO2水平。电化学允许利用电输入来驱动化学反应。由于二氧化碳在动力学上是惰性的,需要高活性的催化剂来充分降低反应势垒,以便获得足以降低电化学CO 2的反应速率。鉴于与CO2的多个电子质子还原相关的反应障碍,甲醛(HC(O)H),甲酸或甲酸(HC(O)OH,HC(O)O-)或更高度还原的形式对于碳,对催化的高选择性也有需求。已探究的催化剂包括溶液中的均相催化剂,固定在表面上的催化剂和非均相催化剂。在均相催化中,还原发生在催化剂扩散到发生多个质子耦合电子转移还原的电极之后。在该领域中有用的催化剂通常是具有有机配体和利用金属和配体氧化还原水平的组合的电子转移性质的过渡金属配合物。作为限制催化剂量的一种方式,在类似于装置的构造中,通过表面结合,在聚合物膜中或在具有与溶液中的催化剂有关的分子结构和电子构造的碳电极表面上,将催化剂添加到导电基材的表面。固定化的均相催化剂在长期的CO2还原循环中会遭受性能损失甚至分解的困扰,但是它们适合进行详细的机械研究。在平行努力中,随着可提供高活性催化表面积的简便合成方法的发展,已详细探索了多相纳米催化剂。它们的高活性和稳定性吸引了广泛的研究,包括可能用于大规模应用。然而,催化反应性向表面的转化产生了新的反应性环境,并使机理细节的阐明和在探索反应途径中鉴定活性位点变得复杂。在此,总结了基于过渡金属配合物催化剂进行CO2电还原的先前研究结果。早期研究表明,具有明确结构的Ru,Ir,Rh和Os过渡金属络合物都能够催化将CO2还原为CO或甲酸。通过化学键,非共价键或聚合将络合物的衍生物表面附接到导电电极上。通过化学控制沉积纳米结构催化剂(如纳米锡,纳米铜和纳米碳)的化学方法,表面结合的概念也已扩展到表面积电极的制备,所有这些均显示出对二氧化碳的高选择性和活性。减少。在我们的演示中,我们以最近的进展和有关电催化在二氧化碳还原中的应用的观点来结束该帐户。
更新日期:2020-01-08
中文翻译:

减少二氧化碳:从均相到多相电催化。
由于世界范围内化石燃料消耗的增加,大气中二氧化碳的含量增加,对环境的影响日益重要。已经提出了可再生的清洁能源,包括风能和太阳能,但是它们是间歇性的,需要高效且可扩展的储能技术。电化学二氧化碳还原反应(CO2RR)在这一领域提供了一种有价值的方法。它结合了太阳能或风能发电与碳基燃料化学键中的能量存储。它可以提供在能量循环中整合碳捕获,利用和存储的方法,同时保持受控的大气CO2水平。电化学允许利用电输入来驱动化学反应。由于二氧化碳在动力学上是惰性的,需要高活性的催化剂来充分降低反应势垒,以便获得足以降低电化学CO 2的反应速率。鉴于与CO2的多个电子质子还原相关的反应障碍,甲醛(HC(O)H),甲酸或甲酸(HC(O)OH,HC(O)O-)或更高度还原的形式对于碳,对催化的高选择性也有需求。已探究的催化剂包括溶液中的均相催化剂,固定在表面上的催化剂和非均相催化剂。在均相催化中,还原发生在催化剂扩散到发生多个质子耦合电子转移还原的电极之后。在该领域中有用的催化剂通常是具有有机配体和利用金属和配体氧化还原水平的组合的电子转移性质的过渡金属配合物。作为限制催化剂量的一种方式,在类似于装置的构造中,通过表面结合,在聚合物膜中或在具有与溶液中的催化剂有关的分子结构和电子构造的碳电极表面上,将催化剂添加到导电基材的表面。固定化的均相催化剂在长期的CO2还原循环中会遭受性能损失甚至分解的困扰,但是它们适合进行详细的机械研究。在平行努力中,随着可提供高活性催化表面积的简便合成方法的发展,已详细探索了多相纳米催化剂。它们的高活性和稳定性吸引了广泛的研究,包括可能用于大规模应用。然而,催化反应性向表面的转化产生了新的反应性环境,并使机理细节的阐明和在探索反应途径中鉴定活性位点变得复杂。在此,总结了基于过渡金属配合物催化剂进行CO2电还原的先前研究结果。早期研究表明,具有明确结构的Ru,Ir,Rh和Os过渡金属络合物都能够催化将CO2还原为CO或甲酸。通过化学键,非共价键或聚合将络合物的衍生物表面附接到导电电极上。通过化学控制沉积纳米结构催化剂(如纳米锡,纳米铜和纳米碳)的化学方法,表面结合的概念也已扩展到表面积电极的制备,所有这些均显示出对二氧化碳的高选择性和活性。减少。在我们的演示中,我们以最近的进展和有关电催化在二氧化碳还原中的应用的观点来结束该帐户。


























 京公网安备 11010802027423号
京公网安备 11010802027423号