当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Clathrate hydrate based approach for concentration of sugar aqueous solution, orange juice and tomato juice: Phase equilibria using a thermodynamic framework
Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.fluid.2020.112460 Mohammad M. Ghiasi , Amir H. Mohammadi , Sohrab Zendehboudi
Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.fluid.2020.112460 Mohammad M. Ghiasi , Amir H. Mohammadi , Sohrab Zendehboudi
|
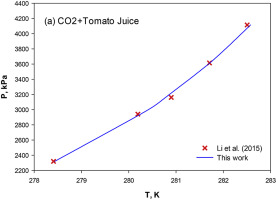
Abstract Utilization of hydrate formation appears to be an efficient technology to facilitate concentration process of various juices in the food industry. This research work examines a thermodynamic approach to simulate the concentration operation of sugar solutions in terms of process feasibility and effectiveness. To attain this objective, the previously presented semi-theoretical framework for exploring the inhibition effects of various salts and alcohols (thermodynamic inhibitors) on the equilibrium stability conditions of methane hydrates was extended to forecast the methane/carbon dioxide hydrate dissociation temperature in the presence of sugar aqueous solutions, orange juice, and tomato juice. The experimental liquid-hydrate-vapor (L-H-V) three-phase equilibrium data of CH4+xylose/xylitol + water and CO2+sucrose/fructose + water systems were gathered from the literature. A new empirical correlation capable of estimating the incipient stability curve of the carbon dioxide hydrate in pure water was developed using the real data reported in the time interval of 1946–2017. Furthermore, the phase equilibria conditions of CO2 hydrate in orange juice and tomato juice were modelled. For all the studied methane and carbon dioxide hydrate systems, the proposed approach reproduces the experimental data with absolute deviations around 0.3 and 0.2 K, respectively, within the temperature, pressure, and solution concentration ranges of the collected data. For CO2+orange and tomato juice hydrate systems, the absolute errors were found to be 0.17 and 0.06, respectively. This research work has potentials to design and operate an efficient-energy strategy for concentrating different types of sugar solutions in the food industry.
中文翻译:

基于包合物水合物浓缩糖水溶液、橙汁和番茄汁的方法:使用热力学框架的相平衡
摘要 利用水合物形成似乎是一种促进食品工业中各种果汁浓缩过程的有效技术。这项研究工作检验了在工艺可行性和有效性方面模拟糖溶液浓缩操作的热力学方法。为了实现这一目标,先前提出的探索各种盐类和醇类(热力学抑制剂)对甲烷水合物平衡稳定性条件的抑制作用的半理论框架被扩展到预测甲烷/二氧化碳水合物存在下的解离温度。糖水溶液、橙汁和番茄汁。CH4+木糖/木糖醇+水和CO2+蔗糖/果糖+水系统的实验液体-水合物-蒸气(LHV)三相平衡数据是从文献中收集的。使用 1946 年至 2017 年时间间隔内报告的真实数据开发了一种新的经验相关性,能够估计纯水中二氧化碳水合物的初始稳定性曲线。此外,还模拟了橙汁和番茄汁中 CO2 水合物的相平衡条件。对于所有研究的甲烷和二氧化碳水合物系统,所提出的方法在所收集数据的温度、压力和溶液浓度范围内分别以大约 0.3 和 0.2 K 的绝对偏差再现了实验数据。对于 CO2+橙子和番茄汁水合物系统,发现绝对误差为 0.17 和 0。06,分别。这项研究工作有可能设计和运行一种高效能源策略,用于在食品工业中浓缩不同类型的糖溶液。
更新日期:2020-05-01
中文翻译:

基于包合物水合物浓缩糖水溶液、橙汁和番茄汁的方法:使用热力学框架的相平衡
摘要 利用水合物形成似乎是一种促进食品工业中各种果汁浓缩过程的有效技术。这项研究工作检验了在工艺可行性和有效性方面模拟糖溶液浓缩操作的热力学方法。为了实现这一目标,先前提出的探索各种盐类和醇类(热力学抑制剂)对甲烷水合物平衡稳定性条件的抑制作用的半理论框架被扩展到预测甲烷/二氧化碳水合物存在下的解离温度。糖水溶液、橙汁和番茄汁。CH4+木糖/木糖醇+水和CO2+蔗糖/果糖+水系统的实验液体-水合物-蒸气(LHV)三相平衡数据是从文献中收集的。使用 1946 年至 2017 年时间间隔内报告的真实数据开发了一种新的经验相关性,能够估计纯水中二氧化碳水合物的初始稳定性曲线。此外,还模拟了橙汁和番茄汁中 CO2 水合物的相平衡条件。对于所有研究的甲烷和二氧化碳水合物系统,所提出的方法在所收集数据的温度、压力和溶液浓度范围内分别以大约 0.3 和 0.2 K 的绝对偏差再现了实验数据。对于 CO2+橙子和番茄汁水合物系统,发现绝对误差为 0.17 和 0。06,分别。这项研究工作有可能设计和运行一种高效能源策略,用于在食品工业中浓缩不同类型的糖溶液。


























 京公网安备 11010802027423号
京公网安备 11010802027423号