当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
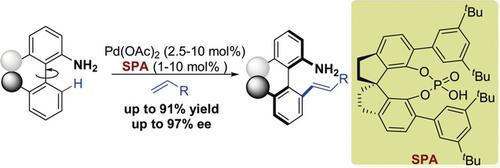
Synthesis of Axially Chiral Biaryl-2-amines by PdII -Catalyzed Free-Amine-Directed Atroposelective C-H Olefination.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-01-07 , DOI: 10.1002/anie.201915674 Bei-Bei Zhan 1 , Lei Wang 1 , Jun Luo 1 , Xu-Feng Lin 1 , Bing-Feng Shi 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-01-07 , DOI: 10.1002/anie.201915674 Bei-Bei Zhan 1 , Lei Wang 1 , Jun Luo 1 , Xu-Feng Lin 1 , Bing-Feng Shi 1
Affiliation
|
A simple and ubiquitously present group, free amine, is used as a directing group to synthesize axially chiral biaryl compounds by PdII -catalyzed atroposelective C-H olefination. A broad range of axially chiral biaryl-2-amines can be obtained in good yields with high enantioselectivities (up to 97 % ee). Chiral spiro phosphoric acid (SPA) proved to be an efficient ligand and the loading could be reduced to 1 mol % without erosion of enantiocontrol in gram-scale synthesis. The resulting axially chiral biaryl-2-amines also provide a platform for the synthesis of a set of chiral ligands.
中文翻译:

PdII催化的游离胺导向的Atroposelective CH Olefination合成轴向手性联芳基-2-胺。
一个简单且普遍存在的基团,即游离胺,被用作通过PdII催化的对映选择性CH烯化反应合成轴向手性联芳基化合物的导向基团。可以以高收率和高对映选择性(高达97%ee)获得多种轴向手性联芳基-2-胺。手性螺环磷酸(SPA)被证明是一种有效的配体,在克级合成中,对映体的控制不会降低其负载量至1 mol%。所得的轴向手性联芳基-2-胺也为合成一组手性配体提供了平台。
更新日期:2020-01-24
中文翻译:

PdII催化的游离胺导向的Atroposelective CH Olefination合成轴向手性联芳基-2-胺。
一个简单且普遍存在的基团,即游离胺,被用作通过PdII催化的对映选择性CH烯化反应合成轴向手性联芳基化合物的导向基团。可以以高收率和高对映选择性(高达97%ee)获得多种轴向手性联芳基-2-胺。手性螺环磷酸(SPA)被证明是一种有效的配体,在克级合成中,对映体的控制不会降低其负载量至1 mol%。所得的轴向手性联芳基-2-胺也为合成一组手性配体提供了平台。


























 京公网安备 11010802027423号
京公网安备 11010802027423号