当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Generation and Reactivity of Amidyl Radicals: Manganese-Mediated Atom-Transfer Reaction.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-01-07 , DOI: 10.1002/anie.201913042 Run-Zhou Liu 1 , Jinxia Li 2 , Jun Sun 1 , Xian-Guan Liu 1 , Shuanglin Qu 2 , Ping Li 1 , Bo Zhang 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-01-07 , DOI: 10.1002/anie.201913042 Run-Zhou Liu 1 , Jinxia Li 2 , Jun Sun 1 , Xian-Guan Liu 1 , Shuanglin Qu 2 , Ping Li 1 , Bo Zhang 1
Affiliation
|
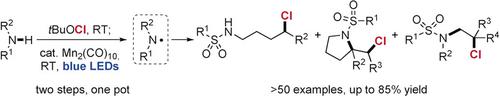
A simple and efficient protocol to generate amidyl radicals from amine functionalities through a manganese-mediated atom-transfer reaction has been developed. This approach employs an earth-abundant and inexpensive manganese complex, Mn2 (CO)10 , as the catalyst and visible light as the energy input. Using this strategy, site-selective chlorination of unactivated C(sp3 )-H bonds of aliphatic amines and intramolecular/intermolecular chloroaminations of unactivated alkenes were readily realized under mild reaction conditions, thus providing efficient access to a range of synthetically valuable alkyl chlorides, chlorinated pyrrolidines, and vicinal chloroamine derivatives. These practical reactions exhibit a broad substrate scope and tolerate a wide array of functional groups, and complex molecules including various marketed drug derivatives.
中文翻译:

酰胺基自由基的产生和反应性:锰介导的原子转移反应。
已经开发了一种简单有效的方案,可通过锰介导的原子转移反应从胺官能团生成酰胺基。该方法采用了一种富含地球和廉价的锰络合物Mn2(CO)10作为催化剂,并使用了可见光作为能量输入。使用这种策略,脂肪族胺的未活化C(sp3)-H键和未活化烯烃的分子内/分子间氯胺的定点氯化很容易在温和的反应条件下实现,因此可以有效地获得一系列有价值的,氯化的烷基氯化物。吡咯烷和邻氯胺衍生物。这些实际反应显示出广泛的底物范围,并能耐受各种官能团以及包括各种市售药物衍生物在内的复杂分子。
更新日期:2020-01-24
中文翻译:

酰胺基自由基的产生和反应性:锰介导的原子转移反应。
已经开发了一种简单有效的方案,可通过锰介导的原子转移反应从胺官能团生成酰胺基。该方法采用了一种富含地球和廉价的锰络合物Mn2(CO)10作为催化剂,并使用了可见光作为能量输入。使用这种策略,脂肪族胺的未活化C(sp3)-H键和未活化烯烃的分子内/分子间氯胺的定点氯化很容易在温和的反应条件下实现,因此可以有效地获得一系列有价值的,氯化的烷基氯化物。吡咯烷和邻氯胺衍生物。这些实际反应显示出广泛的底物范围,并能耐受各种官能团以及包括各种市售药物衍生物在内的复杂分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号