当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemical Causes of Metal Nobleness.
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-01-22 , DOI: 10.1002/cphc.202000013 Kasper P Kepp 1
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-01-22 , DOI: 10.1002/cphc.202000013 Kasper P Kepp 1
Affiliation
|
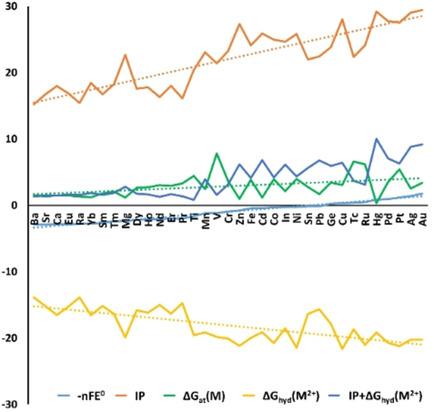
Humans have appreciated the “noble” metals for millennia, yet modern chemistry still struggles with different definitions. Herein, metal nobleness is analyzed using thermochemical cycles including the different bulk, gas, and solution states implied by these definitions. The analysis suggests that metal nobleness mainly reflects inability to fulfil the electron demands of electronegative oxygen. Accordingly, gold is the most noble metal in existence, not because of d‐band properties of the solid state, but because gold's electronegativity is closest to that of oxygen, producing weaker polar covalent bonding. The high electronegativity arises from the effective nuclear charge due to diffuse d‐states, enforced by relativistic effects. This explanation accounts for the activity series, corrosion tendency, and trends in oxygen chemisorption, which other models do not. While gold is the most noble metal, the ranking of Ag, Pt, and Pd depends on the thermochemistry as discussed in detail.
中文翻译:

金属贵金属的化学原因。
几千年来,人类一直在欣赏“贵重”金属,但现代化学仍在为不同的定义而苦苦挣扎。本文中,使用包括这些定义所隐含的不同体积,气体和溶液状态的热化学循环来分析金属贵金属。分析表明,金属贵金属主要反映了不能满足负电性氧对电子的需求。因此,金是存在的最贵重的金属,这不是因为固态的d波段特性,而是因为金的电负性最接近氧,从而产生较弱的极性共价键。高电负性是由于相对论效应强制的扩散d状态导致的有效核电荷所致。该解释说明了活性系列,腐蚀趋势以及氧气化学吸附的趋势,其他型号没有。尽管金是最贵重的金属,但Ag,Pt和Pd的排名取决于详细讨论的热化学。
更新日期:2020-01-22
中文翻译:

金属贵金属的化学原因。
几千年来,人类一直在欣赏“贵重”金属,但现代化学仍在为不同的定义而苦苦挣扎。本文中,使用包括这些定义所隐含的不同体积,气体和溶液状态的热化学循环来分析金属贵金属。分析表明,金属贵金属主要反映了不能满足负电性氧对电子的需求。因此,金是存在的最贵重的金属,这不是因为固态的d波段特性,而是因为金的电负性最接近氧,从而产生较弱的极性共价键。高电负性是由于相对论效应强制的扩散d状态导致的有效核电荷所致。该解释说明了活性系列,腐蚀趋势以及氧气化学吸附的趋势,其他型号没有。尽管金是最贵重的金属,但Ag,Pt和Pd的排名取决于详细讨论的热化学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号