当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Basis for Substrate Specificity and Carbapenemase Activity of OXA-48 Class D β-Lactamase.
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-01-07 , DOI: 10.1021/acsinfecdis.9b00304 Afroza Akhtar 1 , Orville A Pemberton 1 , Yu Chen 1
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-01-07 , DOI: 10.1021/acsinfecdis.9b00304 Afroza Akhtar 1 , Orville A Pemberton 1 , Yu Chen 1
Affiliation
|
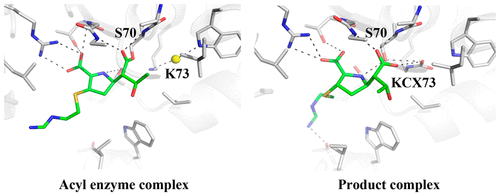
Carbapenem-hydrolyzing class D β-lactamases (CHDLs) are a diverse family of enzymes that are rapidly becoming the predominant cause of bacterial resistance against β-lactam antibiotics in many regions of the world. OXA-48, an atypical member of CHDLs, is one of the most frequently observed in the clinic and exhibits a unique substrate profile. We applied X-ray crystallography to OXA-48 complexes with multiple β-lactam antibiotics to elucidate this enzyme's carbapenemase activity and its preference of imipenem over meropenem and other substrates such as cefotaxime. In particular, we obtained acyl-enzyme complexes of OXA-48 with imipenem, meropenem, faropenem, cefotaxime, and cefoxitin, and a product complex with imipenem. Importantly, the product complex captures a key reaction milestone with the newly generated carboxylate group still in the oxyanion hole, and represents the first such complex with a wild-type serine β-lactamase. A potential hydrogen bond is observed between the two carboxylate groups from the product and the carbamylated Lys73, representing the stage immediately after the breakage of the acyl-enzyme bond where the product carboxylate would be neutral. The placement of the product carboxylate also illustrates the approximate transient location of the deacylation water that has long eluded structural characterization in class D β-lactamases. Additionally, comparing the product complex with the acyl-enzyme intermediates provides new insights into the various mechanisms by which specific side chain groups hinder the access of the deacylation water to the acyl-enzyme linkage, especially in meropenem. Taken together, these data offer valuable information on the substrate specificity of OXA-48 and the catalytic mechanism of CHDLs.
中文翻译:

OXA-48 D类β-内酰胺酶的底物特异性和碳青霉烯酶活性的结构基础。
碳青霉烯水解性D类β-内酰胺酶(CHDLs)是一个多样化的酶家族,在世界许多地区正迅速成为细菌对β-内酰胺抗生素产生耐药性的主要原因。OXA-48是CHDL的非典型成员,是临床上最常观察到的一种,并且具有独特的底物特征。我们对具有多种β-内酰胺抗生素的OXA-48配合物进行了X射线晶体学分析,以阐明该酶的碳青霉烯酶活性及其对亚胺培南的优先于美洛培南和其他底物,如头孢噻肟。特别是,我们获得了OXA-48与亚胺培南,美罗培南,法罗培南,头孢噻肟和头孢西丁的酰基酶复合物,以及与亚胺培南的产物复合物。重要的,该产物配合物捕获了一个关键的反应里程碑,其中新生成的羧酸酯基团仍位于氧阴离子孔中,代表了首个具有野生型丝氨酸β-内酰胺酶的配合物。在产物和氨基甲酰化的Lys73的两个羧酸酯基团之间观察到潜在的氢键,代表酰基-酶键断裂后立即发生的阶段,其中产物羧酸酯将是中性的。产物羧酸盐的位置也说明了在D类β-内酰胺酶中长期存在结构特征的脱酰水的近似瞬时位置。另外,将产物复合物与酰基酶中间体进行比较,为各种机制提供了新的见解,通过这些机制特定的侧链基团会阻碍脱酰水与酰基酶键的接触,特别是在美洛培南中。总之,这些数据提供了有关OXA-48底物特异性和CHDL催化机理的有价值的信息。
更新日期:2020-01-08
中文翻译:

OXA-48 D类β-内酰胺酶的底物特异性和碳青霉烯酶活性的结构基础。
碳青霉烯水解性D类β-内酰胺酶(CHDLs)是一个多样化的酶家族,在世界许多地区正迅速成为细菌对β-内酰胺抗生素产生耐药性的主要原因。OXA-48是CHDL的非典型成员,是临床上最常观察到的一种,并且具有独特的底物特征。我们对具有多种β-内酰胺抗生素的OXA-48配合物进行了X射线晶体学分析,以阐明该酶的碳青霉烯酶活性及其对亚胺培南的优先于美洛培南和其他底物,如头孢噻肟。特别是,我们获得了OXA-48与亚胺培南,美罗培南,法罗培南,头孢噻肟和头孢西丁的酰基酶复合物,以及与亚胺培南的产物复合物。重要的,该产物配合物捕获了一个关键的反应里程碑,其中新生成的羧酸酯基团仍位于氧阴离子孔中,代表了首个具有野生型丝氨酸β-内酰胺酶的配合物。在产物和氨基甲酰化的Lys73的两个羧酸酯基团之间观察到潜在的氢键,代表酰基-酶键断裂后立即发生的阶段,其中产物羧酸酯将是中性的。产物羧酸盐的位置也说明了在D类β-内酰胺酶中长期存在结构特征的脱酰水的近似瞬时位置。另外,将产物复合物与酰基酶中间体进行比较,为各种机制提供了新的见解,通过这些机制特定的侧链基团会阻碍脱酰水与酰基酶键的接触,特别是在美洛培南中。总之,这些数据提供了有关OXA-48底物特异性和CHDL催化机理的有价值的信息。



























 京公网安备 11010802027423号
京公网安备 11010802027423号