当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemoselective Synthesis and Evaluation of β-Oxovinylarsines as an Arsenic Synthetic Precursor
Organometallics ( IF 2.8 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.organomet.9b00587 Wee Shan Tay 1 , Yongxin Li 1 , Yunpeng Lu 1 , Sumod A. Pullarkat 1 , Pak-Hing Leung 1
Organometallics ( IF 2.8 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.organomet.9b00587 Wee Shan Tay 1 , Yongxin Li 1 , Yunpeng Lu 1 , Sumod A. Pullarkat 1 , Pak-Hing Leung 1
Affiliation
|
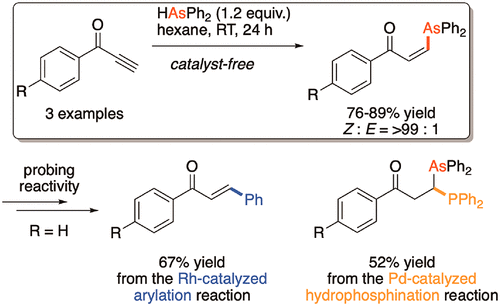
β-Oxovinylarsines are introduced as a stable and highly functionalized bench-top arsenic precursor. Stereoselective syntheses furnished geometrically pure cis- and trans-isomers from the same ynone under different conditions. The organic Michael acceptor backbone allowed β-oxovinylarsines to be easily applied to conjugate-addition reactions, and β-oxovinylarsines were reactive to nucleophiles, electrophiles, and transition metals. Unlike other arsenic precursors which are toxic or volatile or have low reactivity, β-oxovinylarsines bear a well-defined organic backbone that confers both stability and known reactivity. Remarkably, β-oxovinylarsines were also compatible with organometallic catalysts and did not decompose or deactivate the catalyst by chelation.
中文翻译:

砷合成前体β-氧代卵磷脂的化学选择性合成与评价
引入β-氧代ovyylysines作为稳定和高度功能化的台式砷前体。立体选择性合成在不同条件下从相同的炔酮中提供了几何纯的顺式和反式异构体。有机迈克尔受体主链使β-氧代ovyylarsines可以轻松地用于共轭加成反应,并且β-氧代ovyylarsines对亲核试剂,亲电试剂和过渡金属具有反应性。与其他有毒或易挥发的或具有低反应性的砷前体不同,β-氧代ovyylarsines具有定义明确的有机主链,可赋予稳定性和已知的反应性。值得注意的是,β-氧代ovyylarsines也与有机金属催化剂相容,并且不会因螯合作用而分解或失活催化剂。
更新日期:2020-01-08
中文翻译:

砷合成前体β-氧代卵磷脂的化学选择性合成与评价
引入β-氧代ovyylysines作为稳定和高度功能化的台式砷前体。立体选择性合成在不同条件下从相同的炔酮中提供了几何纯的顺式和反式异构体。有机迈克尔受体主链使β-氧代ovyylarsines可以轻松地用于共轭加成反应,并且β-氧代ovyylarsines对亲核试剂,亲电试剂和过渡金属具有反应性。与其他有毒或易挥发的或具有低反应性的砷前体不同,β-氧代ovyylarsines具有定义明确的有机主链,可赋予稳定性和已知的反应性。值得注意的是,β-氧代ovyylarsines也与有机金属催化剂相容,并且不会因螯合作用而分解或失活催化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号