当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydroperoxo double hydrogen bonding: stabilization of hydroperoxo complexes exemplified by triphenylsilicon and triphenylgermanium hydroperoxides
CrystEngComm ( IF 3.1 ) Pub Date : 2020/01/07 , DOI: 10.1039/c9ce01882d Alexander G. Medvedev 1, 2, 3, 4 , Dmitry A. Grishanov 1, 2, 3, 4, 5 , Andrei V. Churakov 1, 2, 3, 4 , Alexey A. Mikhaylov 1, 2, 3, 4 , Ovadia Lev 5, 6, 7, 8, 9 , Petr V. Prikhodchenko 1, 2, 3, 4
CrystEngComm ( IF 3.1 ) Pub Date : 2020/01/07 , DOI: 10.1039/c9ce01882d Alexander G. Medvedev 1, 2, 3, 4 , Dmitry A. Grishanov 1, 2, 3, 4, 5 , Andrei V. Churakov 1, 2, 3, 4 , Alexey A. Mikhaylov 1, 2, 3, 4 , Ovadia Lev 5, 6, 7, 8, 9 , Petr V. Prikhodchenko 1, 2, 3, 4
Affiliation
|
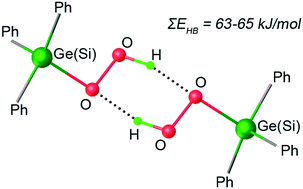
Triphenyl silicon hydroperoxide and its isostructural germanium complex were characterized by single crystal X-ray analysis revealing H-bonding of two triphenylhydroperoxocomplexes, with each hydroperoxo ligand acting as a hydrogen donor and a hydrogen acceptor. Only two other structures with localized protons of hydroperoxo complexes' main group elements (boron and tin) are known (compared to 130 p-block element peroxo compounds) and both exhibit the same hydroperoxo double hydrogen bonding motif. The reaction of the hydroperoxo complexes with triphenylgermanium chloride to give the dinuclear peroxobridged germanium complex demonstrates the higher reactivity of the hydroperoxo moieties compared to the peroxo moiety. DFT calculations provide an estimate of the hydroperoxo double hydrogen bond energies: 62.8 and 63.6 kJ mol−1 for triphenyl silicon, 63.6 and 65 kJ mol−1 for the germanium complex.
中文翻译:

氢过氧双氢键:氢过氧配合物的稳定化,例如三苯基硅和三苯基锗氢过氧化物
通过单晶X射线分析对三苯基氢过氧化硅及其同构锗络合物进行表征,揭示了两个三苯基氢过氧化配合物的氢键,每个氢过氧化配体分别充当氢供体和氢受体。只有其他两个带有氢过氧化物配合物主族元素(硼和锡)的局部质子的结构(与130个p-嵌段元素过氧化合物相比)是已知的,并且它们都显示出相同的氢过氧双氢键基序。氢过氧配合物与三苯基锗氯化物的反应生成双核过氧桥接的锗配合物,表明氢过氧部分与过氧部分相比具有更高的反应性。DFT计算提供了氢过氧双氢键能的估计值:62.8和63.6 kJ mol-1用于三苯硅,63.6和65千焦耳摩尔-1的锗复杂。
更新日期:2020-03-16
中文翻译:

氢过氧双氢键:氢过氧配合物的稳定化,例如三苯基硅和三苯基锗氢过氧化物
通过单晶X射线分析对三苯基氢过氧化硅及其同构锗络合物进行表征,揭示了两个三苯基氢过氧化配合物的氢键,每个氢过氧化配体分别充当氢供体和氢受体。只有其他两个带有氢过氧化物配合物主族元素(硼和锡)的局部质子的结构(与130个p-嵌段元素过氧化合物相比)是已知的,并且它们都显示出相同的氢过氧双氢键基序。氢过氧配合物与三苯基锗氯化物的反应生成双核过氧桥接的锗配合物,表明氢过氧部分与过氧部分相比具有更高的反应性。DFT计算提供了氢过氧双氢键能的估计值:62.8和63.6 kJ mol-1用于三苯硅,63.6和65千焦耳摩尔-1的锗复杂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号