当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The primary photo-dissociation dynamics of amino acids in aqueous solution: breaking the Cα-bond.
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9cp05836b Marlene Møller Madsen 1 , Frank Jensen 1 , Jan Thøgersen 1
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9cp05836b Marlene Møller Madsen 1 , Frank Jensen 1 , Jan Thøgersen 1
Affiliation
|
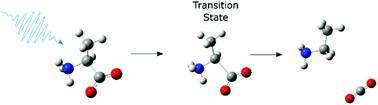
We report a study of the primary photo-dissociation dynamics of aqueous alanine, isoleucine and proline by 200 nm UV pump-IR probe transient absorption spectroscopy. Photo-dissociation of the three amino acids predominantly results in decarboxylation, and 38 ± 7% of the excited alanine, 35 ± 10% of the excited isoleucine and 47 ± 10% of the excited proline zwitterions remain dissociated 100 picoseconds after the excitation. The decarboxylation occurs from a transient intermediate with a lifetime of ∼20 picoseconds to which we assign the excited state of the amino acids based on comparison of the measured and calculated IR spectra, and calculated excited state energy surfaces.
中文翻译:

水溶液中氨基酸的主要光解离动力学:打破Cα键。
我们报告了对丙氨酸,异亮氨酸和脯氨酸水溶液的主要光解离动力学的研究,该动力学通过200 nm紫外泵浦IR探针瞬态吸收光谱法进行。这三个氨基酸的光离解主要导致脱羧,并且在激发后100皮秒保持离解的38±7%的丙氨酸,35±10%的异亮氨酸和47±10%的脯氨酸两性离子离解。脱羧发生在一个瞬态中间体上,该中间体的寿命约为20皮秒,我们根据测得的和计算出的IR光谱以及计算出的激发态能表面,将其分配为氨基酸的激发态。
更新日期:2020-01-13
中文翻译:

水溶液中氨基酸的主要光解离动力学:打破Cα键。
我们报告了对丙氨酸,异亮氨酸和脯氨酸水溶液的主要光解离动力学的研究,该动力学通过200 nm紫外泵浦IR探针瞬态吸收光谱法进行。这三个氨基酸的光离解主要导致脱羧,并且在激发后100皮秒保持离解的38±7%的丙氨酸,35±10%的异亮氨酸和47±10%的脯氨酸两性离子离解。脱羧发生在一个瞬态中间体上,该中间体的寿命约为20皮秒,我们根据测得的和计算出的IR光谱以及计算出的激发态能表面,将其分配为氨基酸的激发态。


























 京公网安备 11010802027423号
京公网安备 11010802027423号