当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adsorption processes on a Pd monolayer-modified Pt(111) electrode
Chemical Science ( IF 8.4 ) Pub Date : 2020/01/07 , DOI: 10.1039/c9sc05307g Xiaoting Chen 1, 2, 3, 4 , Laura P. Granda-Marulanda 1, 2, 3, 4 , Ian T. McCrum 1, 2, 3, 4 , Marc T. M. Koper 1, 2, 3, 4
Chemical Science ( IF 8.4 ) Pub Date : 2020/01/07 , DOI: 10.1039/c9sc05307g Xiaoting Chen 1, 2, 3, 4 , Laura P. Granda-Marulanda 1, 2, 3, 4 , Ian T. McCrum 1, 2, 3, 4 , Marc T. M. Koper 1, 2, 3, 4
Affiliation
|
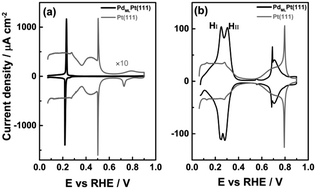
Specific adsorption of anions is an important aspect in surface electrochemistry for its influence on reaction kinetics in either a promoted or inhibited fashion. Perchloric acid is typically considered as an ideal electrolyte for investigating electrocatalytic reactions due to the lack of specific adsorption of the perchlorate anion on several metal electrodes. In this work, cyclic voltammetry and computational methods are combined to investigate the interfacial processes on a Pd monolayer deposited on Pt(111) single crystal electrode in perchloric acid solution. The “hydrogen region” of this PdMLPt(111) surface exhibits two voltammetric peaks: the first “hydrogen peak” at 0.246 VRHE actually involves the replacement of hydrogen by hydroxyl, and the second “hydrogen peak” HII at 0.306 VRHE appears to be the replacement of adsorbed hydroxyl by specific perchlorate adsorption. The two peaks merge into a single peak when a more strongly adsorbed anion, such as sulfate, is involved. Our density functional theory calculations qualitatively support the peak assignment and show that anions generally bind more strongly to the PdMLPt(111) surface than to Pt(111).
中文翻译:

钯单层修饰Pt(111)电极上的吸附过程
阴离子的特异性吸附是表面电化学的一个重要方面,因为它以促进或抑制的方式影响反应动力学。由于高氯酸根阴离子在几个金属电极上缺乏特异性吸附,高氯酸通常被认为是研究电催化反应的理想电解质。在这项工作中,循环伏安法和计算方法相结合,以研究高氯酸溶液中沉积在Pt(111)单晶电极上的Pd单层上的界面过程。Pd ML Pt(111)表面的“氢区域”表现出两个伏安峰:0.246 V RHE处的第一个“氢峰”实际上涉及氢被羟基取代,而第二个“氢峰” HII在0.306 V RHE看来是被特定的高氯酸盐吸附剂替代了吸附的羟基。当涉及到更强吸附的阴离子(例如硫酸盐)时,两个峰合并为一个峰。我们的密度泛函理论计算从质上支持了峰分配,并表明,与Pt(111)相比,阴离子与Pd ML Pt(111)表面的结合通常更牢固。
更新日期:2020-02-13
中文翻译:

钯单层修饰Pt(111)电极上的吸附过程
阴离子的特异性吸附是表面电化学的一个重要方面,因为它以促进或抑制的方式影响反应动力学。由于高氯酸根阴离子在几个金属电极上缺乏特异性吸附,高氯酸通常被认为是研究电催化反应的理想电解质。在这项工作中,循环伏安法和计算方法相结合,以研究高氯酸溶液中沉积在Pt(111)单晶电极上的Pd单层上的界面过程。Pd ML Pt(111)表面的“氢区域”表现出两个伏安峰:0.246 V RHE处的第一个“氢峰”实际上涉及氢被羟基取代,而第二个“氢峰” HII在0.306 V RHE看来是被特定的高氯酸盐吸附剂替代了吸附的羟基。当涉及到更强吸附的阴离子(例如硫酸盐)时,两个峰合并为一个峰。我们的密度泛函理论计算从质上支持了峰分配,并表明,与Pt(111)相比,阴离子与Pd ML Pt(111)表面的结合通常更牢固。



























 京公网安备 11010802027423号
京公网安备 11010802027423号