当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Integrin Subtypes and Nanoscale Ligand Presentation Influence Drug Sensitivity in Cancer Cells.
Nano Letters ( IF 10.8 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.nanolett.9b04607 Jennifer L Young 1, 2 , Ximeng Hua 1, 2 , Heidi Somsel 1, 2 , Florian Reichart 3 , Horst Kessler 3 , Joachim P Spatz 1, 2
Nano Letters ( IF 10.8 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.nanolett.9b04607 Jennifer L Young 1, 2 , Ximeng Hua 1, 2 , Heidi Somsel 1, 2 , Florian Reichart 3 , Horst Kessler 3 , Joachim P Spatz 1, 2
Affiliation
|
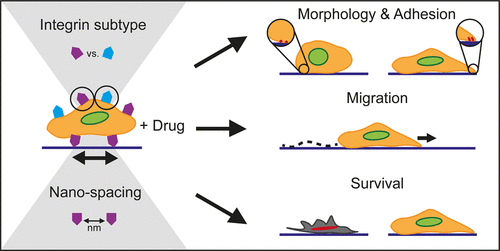
Cancer cell-matrix interactions have been shown to enhance cancer cell survival via the activation of pro-survival signaling pathways. These pathways are initiated at the site of interaction, i.e., integrins, and thus, their inhibition has been the target of therapeutic strategies. Individual roles for fibronectin-binding integrin subtypes αvβ3 and α5β1 have been shown for various cellular processes; however, a systematic comparison of their function in adhesion-dependent chemoresistance is lacking. Here, we utilize integrin subtype-specific peptidomimetics for αvβ3 and α5β1, both as blocking agents on fibronectin-coated surfaces and as surface-immobilized adhesion sites, in order to parse out their role in breast cancer cell survival. Block copolymer micelle nanolithography is utilized to immobilize peptidomimetics onto highly ordered gold nanoparticle arrays with biologically relevant interparticle spacings (35, 50, or 70 nm), thereby providing a platform for ascertaining the dependence of ligand spacing in chemoprotection. We show that several cellular properties-morphology, focal adhesion formation, and migration-are intricately linked to both the integrin subtype and their nanospacing. Importantly, we show that chemotherapeutic drug sensitivity is highly dependent on both parameters, with smaller ligand spacing generally hindering survival. Furthermore, we identify ligand type-specific patterns of drug sensitivity, with enhanced chemosurvival when cells engage αvβ3 vs α5β1 on fibronectin; however, this is heavily reliant on nanoscale spacing, as the opposite is observed when ligands are spaced at 70 nm. These data imply that even nanoscale alterations in extracellular matrix properties have profound effects on cancer cell survival and can thus inform future therapies and drug testing platforms.
中文翻译:

整联蛋白亚型和纳米级配体的表现影响癌细胞中的药物敏感性。
癌细胞与基质之间的相互作用已显示出通过激活生存信号通路来增强癌细胞的存活。这些途径起始于相互作用的部位,即整合素,因此,它们的抑制已成为治疗策略的目标。纤连蛋白结合整联蛋白亚型αvβ3和α5β1在各种细胞过程中的个体作用已显示出来。然而,缺乏对它们在粘附依赖性化学抗性中功能的系统比较。在这里,我们利用αvβ3和α5β1的整联蛋白亚型特异性拟肽作为纤连蛋白涂层表面的封闭剂和表面固定的粘附位点,以分析它们在乳腺癌细胞存活中的作用。嵌段共聚物胶束纳米光刻技术用于将拟肽固定在具有生物学相关的粒子间间距(35、50或70 nm)的高度有序的金纳米粒子阵列上,从而提供了一个平台,可用于确定配体间距在化学保护中的依赖性。我们表明,几个细胞特性-形态,粘着斑形成和迁移-都与整联蛋白亚型和它们的纳米间距错综复杂地联系在一起。重要的是,我们表明化疗药物的敏感性高度依赖于两个参数,较小的配体间距通常会阻碍生存。此外,我们确定了配体类型特异性的药物敏感性模式,当细胞在纤连蛋白上与αvβ3和α5β1结合时,化学存活率提高。但是,这很大程度上取决于纳米级的间距,当配体间隔为70 nm时观察到相反的结果。这些数据表明,甚至细胞外基质性质的纳米级改变对癌细胞的存活也具有深远的影响,因此可以为将来的疗法和药物测试平台提供信息。
更新日期:2020-01-10
中文翻译:

整联蛋白亚型和纳米级配体的表现影响癌细胞中的药物敏感性。
癌细胞与基质之间的相互作用已显示出通过激活生存信号通路来增强癌细胞的存活。这些途径起始于相互作用的部位,即整合素,因此,它们的抑制已成为治疗策略的目标。纤连蛋白结合整联蛋白亚型αvβ3和α5β1在各种细胞过程中的个体作用已显示出来。然而,缺乏对它们在粘附依赖性化学抗性中功能的系统比较。在这里,我们利用αvβ3和α5β1的整联蛋白亚型特异性拟肽作为纤连蛋白涂层表面的封闭剂和表面固定的粘附位点,以分析它们在乳腺癌细胞存活中的作用。嵌段共聚物胶束纳米光刻技术用于将拟肽固定在具有生物学相关的粒子间间距(35、50或70 nm)的高度有序的金纳米粒子阵列上,从而提供了一个平台,可用于确定配体间距在化学保护中的依赖性。我们表明,几个细胞特性-形态,粘着斑形成和迁移-都与整联蛋白亚型和它们的纳米间距错综复杂地联系在一起。重要的是,我们表明化疗药物的敏感性高度依赖于两个参数,较小的配体间距通常会阻碍生存。此外,我们确定了配体类型特异性的药物敏感性模式,当细胞在纤连蛋白上与αvβ3和α5β1结合时,化学存活率提高。但是,这很大程度上取决于纳米级的间距,当配体间隔为70 nm时观察到相反的结果。这些数据表明,甚至细胞外基质性质的纳米级改变对癌细胞的存活也具有深远的影响,因此可以为将来的疗法和药物测试平台提供信息。


























 京公网安备 11010802027423号
京公网安备 11010802027423号