当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthetic Entry to the 2-Azatricyclo[4.3.2.04,9]undecane Ring System via Tropone.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.joc.9b02921 Zaki K Phelan 1 , Philip S Weiss 1 , Yiqun He 1 , Ziyang Guan 1 , Dasan M Thamattoor 2 , Daniel R Griffith 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.joc.9b02921 Zaki K Phelan 1 , Philip S Weiss 1 , Yiqun He 1 , Ziyang Guan 1 , Dasan M Thamattoor 2 , Daniel R Griffith 1
Affiliation
|
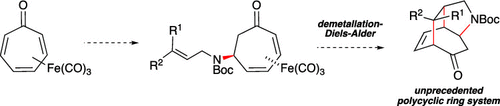
A synthesis of the 2-azatricyclo[4.3.2.04,9]undecane ring system-a hitherto unreported bridged azatricyclic ring system-beginning from tricarbonyl(tropone)iron and allylamine was accomplished in three steps: (1) aza-Michael addition of allylamine to tricarbonyl(tropone)iron; (2) Boc-protection of the resulting secondary amine; and (3) oxidative demetallation leading to a spontaneous intramolecular Diels-Alder reaction. The effect of a variety of parameters on the intramolecular Diels-Alder reaction was investigated, including diene and dienophile substitution patterns and dienophile tether length.
中文翻译:

通过Tropone合成进入2-氮杂三环[4.3.2.04,9]十一烷环系。
由三羰基(托普酮)铁和烯丙胺开始的2-氮杂三环[4.3.2.04,9]十一烷环体系的合成-迄今未报道的桥连氮杂三环体系的合成,分三个步骤进行:(1)烯丙胺的氮杂-迈克尔加成生成三羰基(托乐酮)铁;(2)对所得仲胺进行Boc-保护;(3)氧化脱金属,导致自发的分子内Diels-Alder反应。研究了各种参数对分子内Diels-Alder反应的影响,包括二烯和亲二烯体的取代方式以及亲二烯体的链长。
更新日期:2020-01-17
中文翻译:

通过Tropone合成进入2-氮杂三环[4.3.2.04,9]十一烷环系。
由三羰基(托普酮)铁和烯丙胺开始的2-氮杂三环[4.3.2.04,9]十一烷环体系的合成-迄今未报道的桥连氮杂三环体系的合成,分三个步骤进行:(1)烯丙胺的氮杂-迈克尔加成生成三羰基(托乐酮)铁;(2)对所得仲胺进行Boc-保护;(3)氧化脱金属,导致自发的分子内Diels-Alder反应。研究了各种参数对分子内Diels-Alder反应的影响,包括二烯和亲二烯体的取代方式以及亲二烯体的链长。



























 京公网安备 11010802027423号
京公网安备 11010802027423号