当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Activity-Based Methionine Bioconjugation Approach To Developing Proximity-Activated Imaging Reporters.
ACS Central Science ( IF 18.2 ) Pub Date : 2020-01-07 , DOI: 10.1021/acscentsci.9b01038 Jun Ohata 1 , Lakshmi Krishnamoorthy 1 , Monica A Gonzalez 1 , Tong Xiao 1 , Diana A Iovan 1 , F Dean Toste 1 , Evan W Miller 1 , Christopher J Chang 1
ACS Central Science ( IF 18.2 ) Pub Date : 2020-01-07 , DOI: 10.1021/acscentsci.9b01038 Jun Ohata 1 , Lakshmi Krishnamoorthy 1 , Monica A Gonzalez 1 , Tong Xiao 1 , Diana A Iovan 1 , F Dean Toste 1 , Evan W Miller 1 , Christopher J Chang 1
Affiliation
|
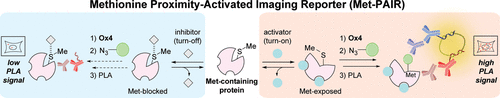
Chemical probes that report on protein activity, rather than protein abundance, with spatial and temporal resolution can enable studies of their native function in biological contexts as well as provide opportunities for developing new types of biochemical reporters. Here we present a sensing platform, termed proximity-activated imaging reporter (PAIR), which combines activity-based methionine bioconjugation and antibody labeling with proximity-dependent oligonucleotide-based amplification to monitor dynamic changes of a given analyte in cells and animals through context-dependent methionine labeling of specific protein targets. We establish this PAIR method to develop sensors for imaging reactive oxygen species (ROS) and calcium ions through oxaziridine-directed labeling of reactive methionine residues on β-actin and calmodulin (CaM), respectively, where the extent of methionine bioconjugation on these protein targets can serve as an indicator of oxidative stress or calcium status. In particular, application of PAIR to activity-based CaM detection provides a method for imaging integrated calcium activity in both in vitro cell and in vivo zebrafish models. By relying on native protein biochemistry, PAIR enables redox and metal imaging without introduction of external small molecules or genetically encoded indicators that can potentially buffer the natural/existing pools. This approach can be potentially generalized to target a broader range of analytes by pairing appropriate activity-based protein probes with protein detection reagents in a proximity-driven manner, providing a starting point not only for designing new sensors but also for monitoring endogenous activity of specific protein targets in biological specimens with spatial and temporal fidelity.
中文翻译:

一种基于活性的蛋氨酸生物共轭方法,以开发接近激活的成像记者。
以空间和时间分辨率报告蛋白质活性而不是蛋白质丰度的化学探针可以在生物学背景下研究其天然功能,并为开发新型生化报告基因提供机会。在这里,我们提供了一个感应平台,称为邻近激活成像报告仪(PAIR),该平台将基于活性的蛋氨酸生物缀合和抗体标记与基于邻近的寡核苷酸的扩增相结合,以通过环境监测细胞和动物中给定分析物的动态变化。依赖的蛋氨酸标记特定的蛋白质靶标。我们建立了这种PAIR方法,以开发通过氧氮丙啶定向标记β-肌动蛋白和钙调蛋白(CaM)上的反应性蛋氨酸残基来成像反应性氧物种(ROS)和钙离子的传感器,分别在这些蛋白质靶标上甲硫氨酸生物共轭程度可作为氧化应激或钙状态的指标。特别地,将PAIR应用于基于活性的CaM检测提供了一种在体外细胞和体内斑马鱼模型中对整合钙活性进行成像的方法。通过依赖天然蛋白质生物化学,PAIR可以实现氧化还原和金属成像,而无需引入可能缓冲天然/现有池的外部小分子或遗传编码指示剂。通过以接近驱动的方式将适当的基于活性的蛋白质探针与蛋白质检测试剂配对,该方法可以潜在地推广到更广泛的分析物目标,
更新日期:2020-01-23
中文翻译:

一种基于活性的蛋氨酸生物共轭方法,以开发接近激活的成像记者。
以空间和时间分辨率报告蛋白质活性而不是蛋白质丰度的化学探针可以在生物学背景下研究其天然功能,并为开发新型生化报告基因提供机会。在这里,我们提供了一个感应平台,称为邻近激活成像报告仪(PAIR),该平台将基于活性的蛋氨酸生物缀合和抗体标记与基于邻近的寡核苷酸的扩增相结合,以通过环境监测细胞和动物中给定分析物的动态变化。依赖的蛋氨酸标记特定的蛋白质靶标。我们建立了这种PAIR方法,以开发通过氧氮丙啶定向标记β-肌动蛋白和钙调蛋白(CaM)上的反应性蛋氨酸残基来成像反应性氧物种(ROS)和钙离子的传感器,分别在这些蛋白质靶标上甲硫氨酸生物共轭程度可作为氧化应激或钙状态的指标。特别地,将PAIR应用于基于活性的CaM检测提供了一种在体外细胞和体内斑马鱼模型中对整合钙活性进行成像的方法。通过依赖天然蛋白质生物化学,PAIR可以实现氧化还原和金属成像,而无需引入可能缓冲天然/现有池的外部小分子或遗传编码指示剂。通过以接近驱动的方式将适当的基于活性的蛋白质探针与蛋白质检测试剂配对,该方法可以潜在地推广到更广泛的分析物目标,



























 京公网安备 11010802027423号
京公网安备 11010802027423号