当前位置:
X-MOL 学术
›
Arch. Biochem. Biophys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phenylglyoxal inhibition of the mitochondrial F1FO-ATPase activated by Mg2+ or by Ca2+ provides clues on the mitochondrial permeability transition pore.
Archives of Biochemistry and Biophysics ( IF 3.9 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.abb.2020.108258 Cristina Algieri 1 , Fabiana Trombetti 1 , Alessandra Pagliarani 1 , Vittoria Ventrella 1 , Salvatore Nesci 1
Archives of Biochemistry and Biophysics ( IF 3.9 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.abb.2020.108258 Cristina Algieri 1 , Fabiana Trombetti 1 , Alessandra Pagliarani 1 , Vittoria Ventrella 1 , Salvatore Nesci 1
Affiliation
|
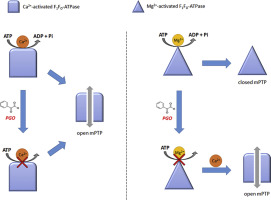
Phenylglyoxal (PGO), known to cause post-translational modifications of Arg residues, was used to highlight the role of arginine residues of the F1FO-ATPase, which may be crucial to yield the mitochondrial permeability transition pore (mPTP). In swine heart mitochondria PGO inhibits ATP hydrolysis by the F1FO-ATPase either sustained by the natural cofactor Mg2+ or by Ca2+ by a similar uncompetitive inhibition mechanism, namely the tertiary complex (ESI) only forms when the ATP substrate is already bound to the enzyme, and with similar strength, as shown by the similar K'i values (0.82 ± 0.07 mM in presence of Mg2+ and 0.64 ± 0.05 mM in the presence of Ca2+). Multiple inhibitor analysis indicates that features of the F1 catalytic sites and/or the FO proton binding sites are apparently unaffected by PGO. However, PGO and F1 or FO inhibitors can bind the enzyme combine simultaneously. However they mutually hinder to bind the Mg2+-activated F1FO-ATPase, whereas they do not mutually exclude to bind the Ca2+-activated F1FO-ATPase. The putative formation of PGO-arginine adducts, and the consequent spatial rearrangement in the enzyme structure, inhibits the F1FO-ATPase activity but, as shown by the calcium retention capacity evaluation in intact mitochondria, apparently favours the mPTP formation.
中文翻译:

苯乙二醛对Mg2 +或Ca2 +激活的线粒体F1FO-ATPase的抑制作用为线粒体通透性过渡孔提供了线索。
苯乙二醛(PGO)已知会引起Arg残基的翻译后修饰,用于强调F1FO-ATPase精氨酸残基的作用,这对于产生线粒体通透性转换孔(mPTP)可能至关重要。在猪心脏线粒体中,PGO通过F1FO-ATPase通过天然辅因子Mg2 +或Ca2 +维持的F1FO-ATPase通过类似的非竞争性抑制机制来抑制ATP水解,即只有当ATP底物已经与该酶结合时才形成三级复合物(ESI),并且具有相似的强度,如相似的K'i值所示(在Mg2 +存在下为0.82±0.07 mM,在Ca2 +存在下为0.64±0.05 mM)。多种抑制剂分析表明,F1催化位点和/或FO质子结合位点的特征显然不受PGO影响。然而,PGO和F1或FO抑制剂可以同时结合酶结合物。然而,它们相互阻碍结合Mg2 +激活的F1FO-ATPase,而它们并不相互排斥以结合Ca2 +激活的F1FO-ATPase。推定的PGO-精氨酸加合物的形成以及随之而来的酶结构空间重排抑制了F1FO-ATPase的活性,但如完整线粒体中钙保留能力的评估所示,显然有利于mPTP的形成。
更新日期:2020-01-07
中文翻译:

苯乙二醛对Mg2 +或Ca2 +激活的线粒体F1FO-ATPase的抑制作用为线粒体通透性过渡孔提供了线索。
苯乙二醛(PGO)已知会引起Arg残基的翻译后修饰,用于强调F1FO-ATPase精氨酸残基的作用,这对于产生线粒体通透性转换孔(mPTP)可能至关重要。在猪心脏线粒体中,PGO通过F1FO-ATPase通过天然辅因子Mg2 +或Ca2 +维持的F1FO-ATPase通过类似的非竞争性抑制机制来抑制ATP水解,即只有当ATP底物已经与该酶结合时才形成三级复合物(ESI),并且具有相似的强度,如相似的K'i值所示(在Mg2 +存在下为0.82±0.07 mM,在Ca2 +存在下为0.64±0.05 mM)。多种抑制剂分析表明,F1催化位点和/或FO质子结合位点的特征显然不受PGO影响。然而,PGO和F1或FO抑制剂可以同时结合酶结合物。然而,它们相互阻碍结合Mg2 +激活的F1FO-ATPase,而它们并不相互排斥以结合Ca2 +激活的F1FO-ATPase。推定的PGO-精氨酸加合物的形成以及随之而来的酶结构空间重排抑制了F1FO-ATPase的活性,但如完整线粒体中钙保留能力的评估所示,显然有利于mPTP的形成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号