当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Proton Wire Mediates Proton Coupled Electron Transfer from Hydroxyurea and Other Hydroxamic Acids to Tyrosyl Radical in Class Ia Ribonucleotide Reductase.
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.jpcb.9b08587 Adam R Offenbacher 1, 2 , Bridgette A Barry 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.jpcb.9b08587 Adam R Offenbacher 1, 2 , Bridgette A Barry 1
Affiliation
|
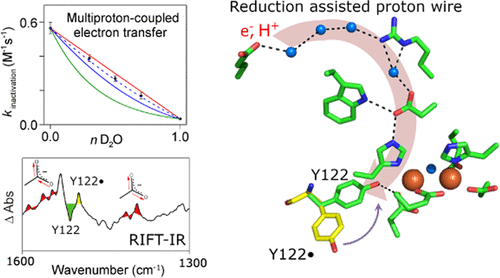
Proton-coupled electron transfer (PCET) is fundamental to many important biological reactions, including solar energy conversion and DNA synthesis. For example, class Ia ribonucleotide reductases (RNRs) contain a tyrosyl radical-diiron cofactor with one aspartate ligand, D84. The tyrosyl radical, Y122•, in the β2 subunit acts as a radical initiator and oxidizes an active site cysteine in the α2 subunit. A transient quaternary α2/β2 complex is induced by substrate and effector binding. The hydroxamic acid, hydroxyurea (HU), reduces Y122• in a PCET reaction involving an electron and proton. This reaction is associated with the loss of activity, a conformational change at Y122, and a change in hydrogen bonding to the Fe1 ligand, D84. Here, we use isotopic labeling, solvent isotope exchange, proton inventories, and reaction-induced Fourier transform infrared (RIFT-IR) spectroscopy to show that the PCET reactions of hydroxamic acids are associated with a characteristic spectrum, which is assignable to electrostatic changes at nonligating aspartate residues. Notably, RIFT-IR spectroscopy reveals this characteristic spectrum when the effects of HU, hydroxylamine, and N-methylhydroxylamine are compared. A large solvent isotope effect is observed for each of the hydroxamic acid reactions, and proton inventories predict that the reactions are associated with the transfer of multiple protons in the transition state. The reduction of Y122• with 4-methoxyphenol does not lead to these characteristic carboxylate shifts and is associated with only a small solvent isotope effect. In addition to studies of the effects of hydroxamic acids on β2 alone, the reactions involving the quaternary α2β2 complex were also investigated. HU treatment of the quaternary complex, α2/β2/ATP/CDP, leads to a similar carboxylate shift spectrum, as observed with β2 alone. The use of globally labeled 13C chimeras (13C α2, 13C β2) confirms the assignment. Because the spectrum is sensitive to 13C β2 labeling, but not 13C α2 labeling, the quaternary complex spectrum is assigned to electrostatic changes in β2 carboxylate groups. Examination of the β2 X-ray structure reveals a hydrogen-bonded network leading from the protein surface to Y122. This predicted network includes nonligating aspartates, glutamate ligands to the iron cluster, and predicted crystallographically resolved water molecules. The network is similar when class Ia RNR structures from Escherichia coli, human, and mouse are compared. We propose that the PCET reactions of hydroxamic acids are mediated by a hydrogen-bonded proton wire in the β2 subunit.
中文翻译:

质子丝在Ia类核糖核苷酸还原酶中介导质子耦合电子从羟基脲和其他异羟肟酸到酪氨酰自由基的转移。
质子耦合电子转移(PCET)是许多重要生物反应(包括太阳能转化和DNA合成)的基础。例如,Ia类核糖核苷酸还原酶(RNR)包含带有一个天冬氨酸配体D84的酪氨酰基-二铁辅因子。β2亚基中的酪氨酸基团Y122•充当自由基引发剂,并氧化α2亚基中的活性位点半胱氨酸。底物和效应物的结合可诱导瞬时的季铵盐α2/β2复合物。异羟肟酸羟基脲(HU)在涉及电子和质子的PCET反应中还原Y122•。该反应与活性丧失,Y122的构象变化以及与Fe1配体D84的氢键变化有关。在这里,我们使用同位素标记,溶剂同位素交换,质子清单,和反应诱导的傅立叶变换红外光谱(RIFT-IR),表明异羟肟酸的PCET反应与特征光谱有关,该特征光谱可归因于未连接的天冬氨酸残基的静电变化。值得注意的是,当比较HU,羟胺和N-甲基羟胺的影响时,RIFT-IR光谱揭示了该特征光谱。对于每个异羟肟酸反应观察到较大的溶剂同位素效应,质子清单预测该反应与过渡态中多个质子的转移相关。用4-甲氧基苯酚还原Y122•不会导致这些特征性的羧酸盐转移,并且仅与较小的溶剂同位素效应有关。除了研究异羟肟酸对β2的影响外,还研究了涉及季α2β2配合物的反应。如单独使用β2所观察到的,对四元络合物α2/β2/ ATP / CDP的HU处理会产生相似的羧酸盐移动光谱。使用全局标记的13C嵌合体(13Cα2,13Cβ2)确认分配。由于该光谱对13Cβ2标记敏感,但对13Cα2标记不敏感,因此将四元络合物光谱分配给β2羧酸酯基团中的静电变化。对β2X射线结构的检查揭示了从蛋白质表面通向Y122的氢键网络。该预测网络包括未连接的天冬氨酸,谷氨酸配体与铁簇,以及预测的晶体学分辨的水分子。比较来自大肠杆菌,人和小鼠的Ia类RNR结构时,网络相似。
更新日期:2020-01-07
中文翻译:

质子丝在Ia类核糖核苷酸还原酶中介导质子耦合电子从羟基脲和其他异羟肟酸到酪氨酰自由基的转移。
质子耦合电子转移(PCET)是许多重要生物反应(包括太阳能转化和DNA合成)的基础。例如,Ia类核糖核苷酸还原酶(RNR)包含带有一个天冬氨酸配体D84的酪氨酰基-二铁辅因子。β2亚基中的酪氨酸基团Y122•充当自由基引发剂,并氧化α2亚基中的活性位点半胱氨酸。底物和效应物的结合可诱导瞬时的季铵盐α2/β2复合物。异羟肟酸羟基脲(HU)在涉及电子和质子的PCET反应中还原Y122•。该反应与活性丧失,Y122的构象变化以及与Fe1配体D84的氢键变化有关。在这里,我们使用同位素标记,溶剂同位素交换,质子清单,和反应诱导的傅立叶变换红外光谱(RIFT-IR),表明异羟肟酸的PCET反应与特征光谱有关,该特征光谱可归因于未连接的天冬氨酸残基的静电变化。值得注意的是,当比较HU,羟胺和N-甲基羟胺的影响时,RIFT-IR光谱揭示了该特征光谱。对于每个异羟肟酸反应观察到较大的溶剂同位素效应,质子清单预测该反应与过渡态中多个质子的转移相关。用4-甲氧基苯酚还原Y122•不会导致这些特征性的羧酸盐转移,并且仅与较小的溶剂同位素效应有关。除了研究异羟肟酸对β2的影响外,还研究了涉及季α2β2配合物的反应。如单独使用β2所观察到的,对四元络合物α2/β2/ ATP / CDP的HU处理会产生相似的羧酸盐移动光谱。使用全局标记的13C嵌合体(13Cα2,13Cβ2)确认分配。由于该光谱对13Cβ2标记敏感,但对13Cα2标记不敏感,因此将四元络合物光谱分配给β2羧酸酯基团中的静电变化。对β2X射线结构的检查揭示了从蛋白质表面通向Y122的氢键网络。该预测网络包括未连接的天冬氨酸,谷氨酸配体与铁簇,以及预测的晶体学分辨的水分子。比较来自大肠杆菌,人和小鼠的Ia类RNR结构时,网络相似。


























 京公网安备 11010802027423号
京公网安备 11010802027423号