当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
X-rays-optimized delivery of radiolabeled albumin for cancer theranostics.
Biomaterials ( IF 14.0 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.biomaterials.2020.119764 Xuan Yi 1 , Hailin Zhou 2 , Zheng Zhang 2 , Saisai Xiong 2 , Kai Yang 2
Biomaterials ( IF 14.0 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.biomaterials.2020.119764 Xuan Yi 1 , Hailin Zhou 2 , Zheng Zhang 2 , Saisai Xiong 2 , Kai Yang 2
Affiliation
|
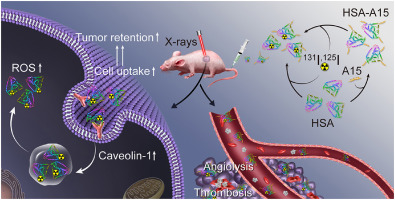
Exploiting the specific biological behaviors of the metabolizable nano-drugs assisted by X-rays exposure will be benefit for the optimization of radiotherapy-based combination therapy. Herein, Human serum albumin (HSA) nanoparticle, a familiar and metabolizable nanomaterial, is selected to investigate the changes of tumor accumulation and retention under X-rays exposure. Caveolin-1, an important protein which has positive correlation with cell uptake of nanomaterials, is expressed increasingly under X-rays exposure, resulting the enhanced cell uptake and prolonged tumor retention of HSA nanoparticles. After being labeled by radioactive iodine-125, HSA could be used for SPECT/CT imaging of mice. Moreover, it discovered that 125I-HSA nanoparticles possess much longer-time retention time in pre-irradiated tumor than that of controlled tumor. Using this strategy, the therapeutic efficiency of 131I-HSA injected mice after irradiating their tumors by X-rays is better than that of opposite sequence treated mice. In order to further improve the targeting ability of HSA, GNQEQVSPLTLLKXC peptide (A15) is conjugated to HSA nanoparticles for targeting the thrombosis in the tumor tissue triggered by X-rays exposure, realizing the high tumor accumulation of 131I-HSA assisted by X-rays exposure. Therefore, taking advantage of the increased expression of Caveolin-1 and the induced thrombosis under X-rays exposure, we optimized the delivery of radiolabeled HSA via enhancing the cell uptake and prolonging tumor retention of HSA for cancer combination therapy. Our work make contribution to guide the clinical albumin based combination therapy.
中文翻译:

X射线优化的放射标记白蛋白的递送,用于癌症治疗学。
在X射线照射辅助下开发可代谢纳米药物的特定生物学行为将有利于优化基于放射疗法的联合疗法。在本文中,选择人类血清白蛋白(HSA)纳米颗粒(一种熟悉且可代谢的纳米材料)来研究X射线暴露下肿瘤堆积和保留的变化。Caveolin-1是一种重要的蛋白质,与纳米材料的细胞摄取呈正相关,在X射线照射下越来越多地表达,从而提高了HSA纳米粒子的细胞摄取并延长了其肿瘤保留时间。经放射性碘125标记后,HSA可用于小鼠的SPECT / CT成像。此外,发现125 I-HSA纳米粒子在预辐照肿瘤中的保留时间比对照肿瘤要长得多。使用这种策略,用X射线照射131I-HSA注射的小鼠的肿瘤后,其治疗效果要好于相反序列治疗的小鼠。为了进一步提高HSA的靶向能力,将GNQEQVSPLTLLKXC肽(A15)与HSA纳米颗粒偶联,以靶向X射线暴露触发的肿瘤组织中的血栓形成,从而在X射线辅助下实现131I-HSA的高肿瘤蓄积接触。因此,利用X射线暴露下Caveolin-1表达的增加和血栓形成的诱导,我们通过增强细胞吸收和延长HSA在肿瘤联合治疗中的肿瘤保留时间,优化了放射性标记HSA的递送。我们的工作为指导基于临床白蛋白的联合疗法做出了贡献。X射线照射131I-HSA注射的小鼠的肿瘤的治疗效果要好于相反序列治疗的小鼠。为了进一步提高HSA的靶向能力,将GNQEQVSPLTLLKXC肽(A15)与HSA纳米颗粒偶联,以靶向X射线暴露触发的肿瘤组织中的血栓形成,从而在X射线辅助下实现131I-HSA的高肿瘤蓄积接触。因此,利用X射线暴露下Caveolin-1表达的增加和血栓形成的诱导,我们通过增强细胞吸收和延长HSA在肿瘤联合治疗中的肿瘤保留时间,优化了放射性标记HSA的递送。我们的工作为指导临床基于白蛋白的联合疗法做出了贡献。X射线照射131I-HSA注射的小鼠的肿瘤的治疗效果要好于相反序列治疗的小鼠。为了进一步提高HSA的靶向能力,将GNQEQVSPLTLLKXC肽(A15)与HSA纳米颗粒偶联,以靶向X射线暴露触发的肿瘤组织中的血栓形成,从而在X射线辅助下实现131I-HSA的高肿瘤蓄积接触。因此,利用X射线暴露下Caveolin-1表达的增加和血栓形成的诱导,我们通过增强细胞吸收和延长HSA在肿瘤联合治疗中的肿瘤保留时间,优化了放射性标记HSA的递送。我们的工作为指导临床基于白蛋白的联合疗法做出了贡献。
更新日期:2020-01-07
中文翻译:

X射线优化的放射标记白蛋白的递送,用于癌症治疗学。
在X射线照射辅助下开发可代谢纳米药物的特定生物学行为将有利于优化基于放射疗法的联合疗法。在本文中,选择人类血清白蛋白(HSA)纳米颗粒(一种熟悉且可代谢的纳米材料)来研究X射线暴露下肿瘤堆积和保留的变化。Caveolin-1是一种重要的蛋白质,与纳米材料的细胞摄取呈正相关,在X射线照射下越来越多地表达,从而提高了HSA纳米粒子的细胞摄取并延长了其肿瘤保留时间。经放射性碘125标记后,HSA可用于小鼠的SPECT / CT成像。此外,发现125 I-HSA纳米粒子在预辐照肿瘤中的保留时间比对照肿瘤要长得多。使用这种策略,用X射线照射131I-HSA注射的小鼠的肿瘤后,其治疗效果要好于相反序列治疗的小鼠。为了进一步提高HSA的靶向能力,将GNQEQVSPLTLLKXC肽(A15)与HSA纳米颗粒偶联,以靶向X射线暴露触发的肿瘤组织中的血栓形成,从而在X射线辅助下实现131I-HSA的高肿瘤蓄积接触。因此,利用X射线暴露下Caveolin-1表达的增加和血栓形成的诱导,我们通过增强细胞吸收和延长HSA在肿瘤联合治疗中的肿瘤保留时间,优化了放射性标记HSA的递送。我们的工作为指导基于临床白蛋白的联合疗法做出了贡献。X射线照射131I-HSA注射的小鼠的肿瘤的治疗效果要好于相反序列治疗的小鼠。为了进一步提高HSA的靶向能力,将GNQEQVSPLTLLKXC肽(A15)与HSA纳米颗粒偶联,以靶向X射线暴露触发的肿瘤组织中的血栓形成,从而在X射线辅助下实现131I-HSA的高肿瘤蓄积接触。因此,利用X射线暴露下Caveolin-1表达的增加和血栓形成的诱导,我们通过增强细胞吸收和延长HSA在肿瘤联合治疗中的肿瘤保留时间,优化了放射性标记HSA的递送。我们的工作为指导临床基于白蛋白的联合疗法做出了贡献。X射线照射131I-HSA注射的小鼠的肿瘤的治疗效果要好于相反序列治疗的小鼠。为了进一步提高HSA的靶向能力,将GNQEQVSPLTLLKXC肽(A15)与HSA纳米颗粒偶联,以靶向X射线暴露触发的肿瘤组织中的血栓形成,从而在X射线辅助下实现131I-HSA的高肿瘤蓄积接触。因此,利用X射线暴露下Caveolin-1表达的增加和血栓形成的诱导,我们通过增强细胞吸收和延长HSA在肿瘤联合治疗中的肿瘤保留时间,优化了放射性标记HSA的递送。我们的工作为指导临床基于白蛋白的联合疗法做出了贡献。


























 京公网安备 11010802027423号
京公网安备 11010802027423号