当前位置:
X-MOL 学术
›
Eur. J. Pharm. Biopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nanoparticles carrying fingolimod and methotrexate enables targeted induction of apoptosis and immobilization of invasive thyroid cancer.
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.9 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.ejpb.2019.12.015 E Niemelä 1 , D Desai 2 , R Niemi 3 , M Doroszko 4 , E Özliseli 2 , K Kemppainen 3 , N A Rahman 5 , C Sahlgren 6 , K Törnquist 7 , J E Eriksson 1 , J M Rosenholm 2
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.9 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.ejpb.2019.12.015 E Niemelä 1 , D Desai 2 , R Niemi 3 , M Doroszko 4 , E Özliseli 2 , K Kemppainen 3 , N A Rahman 5 , C Sahlgren 6 , K Törnquist 7 , J E Eriksson 1 , J M Rosenholm 2
Affiliation
|
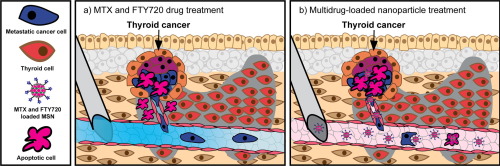
Metastatic tumors are the main cause of cancer-related death, as the invading cancer cells disrupt normal functions of distant organs and are nearly impossible to eradicate by traditional cancer therapeutics. This is of special concern when the cancer has created multiple metastases and extensive surgery would be too dangerous to execute. Therefore, combination chemotherapy is often the selected treatment form. However, drug cocktails often have severe adverse effects on healthy cells, whereby the development of targeted drug delivery could minimize side-effects of drugs and increase the efficacy of the combination therapy. In this study, we utilized the folate antagonist methotrexate (MTX) as targeting ligand conjugated onto mesoporous silica nanoparticles (MSNs) for selective eradication of folate receptor-expressing invasive thyroid cancer cells. The MSNs was subsequently loaded with the drug fingolimod (FTY720), which has previously been shown to efficiently inhibit proliferation and invasion of aggressive thyroid cancer cells. To assess the efficiency of our carrier system, comprehensive in vitro methods were employed; including flow cytometry, confocal microscopy, viability assays, invasion assay, and label-free imaging techniques. The in vitro results show that MTX-conjugated and FTY720-loaded MSNs potently attenuated both the proliferation and invasion of the cancerous thyroid cells while keeping the off-target effects in normal thyroid cells reasonably low. For a more physiologically relevant in vivo approach we utilized the chick chorioallantoic membrane (CAM) assay, showing decreased invasive behavior of the thyroid derived xenografts and an increased necrotic phenotype compared to tumors that received the free drug cocktail. Thus, the developed multidrug-loaded MSNs effectively induced apoptosis and immobilization of invasive thyroid cancer cells, and could potentially be used as a carrier system for targeted drug delivery for the treatment of diverse forms of aggressive cancers that expresses folate receptors.
中文翻译:

携带芬戈莫德和甲氨蝶呤的纳米颗粒能够靶向诱导凋亡和浸润性甲状腺癌的固定化。
转移性肿瘤是与癌症有关的死亡的主要原因,因为入侵的癌细胞会破坏远处器官的正常功能,并且几乎不可能通过传统的癌症治疗方法根除。当癌症产生了多个转移灶并且进行广泛的手术实施起来过于危险时,这尤其值得关注。因此,联合化疗通常是选择的治疗形式。然而,药物混合物通常对健康细胞具有严重的不利影响,由此靶向药物递送的发展可以使药物的副作用最小化并增加联合疗法的功效。在这个研究中,我们利用叶酸拮抗剂甲氨蝶呤(MTX)作为偶联到中孔二氧化硅纳米粒子(MSNs)上的靶向配体,选择性消除了表达叶酸受体的侵袭性甲状腺癌细胞。随后,MSN装载了芬戈莫德(FTY720)药物,该药物先前已被证明可有效抑制侵袭性甲状腺癌细胞的增殖和侵袭。为了评估我们的载体系统的效率,我们采用了综合的体外方法。包括流式细胞仪,共聚焦显微镜,活力测定,侵袭测定和无标记成像技术。体外结果显示,MTX偶联和FTY720偶联的MSN能够有效减弱甲状腺癌细胞的增殖和侵袭,同时保持正常甲状腺细胞的脱靶效应相当低。对于更具生理相关性的体内方法,我们使用了鸡绒膜尿囊膜(CAM)分析,与接受游离药物混合物的肿瘤相比,该方法显示出甲状腺衍生异种移植物的侵袭行为减少,坏死表型增加。因此,已开发的载有多种药物的MSN可有效诱导甲状腺侵袭性癌细胞的凋亡和固定化,并有可能用作靶向药物递送的载体系统,以治疗表达叶酸受体的多种形式的侵袭性癌症。
更新日期:2020-01-07
中文翻译:

携带芬戈莫德和甲氨蝶呤的纳米颗粒能够靶向诱导凋亡和浸润性甲状腺癌的固定化。
转移性肿瘤是与癌症有关的死亡的主要原因,因为入侵的癌细胞会破坏远处器官的正常功能,并且几乎不可能通过传统的癌症治疗方法根除。当癌症产生了多个转移灶并且进行广泛的手术实施起来过于危险时,这尤其值得关注。因此,联合化疗通常是选择的治疗形式。然而,药物混合物通常对健康细胞具有严重的不利影响,由此靶向药物递送的发展可以使药物的副作用最小化并增加联合疗法的功效。在这个研究中,我们利用叶酸拮抗剂甲氨蝶呤(MTX)作为偶联到中孔二氧化硅纳米粒子(MSNs)上的靶向配体,选择性消除了表达叶酸受体的侵袭性甲状腺癌细胞。随后,MSN装载了芬戈莫德(FTY720)药物,该药物先前已被证明可有效抑制侵袭性甲状腺癌细胞的增殖和侵袭。为了评估我们的载体系统的效率,我们采用了综合的体外方法。包括流式细胞仪,共聚焦显微镜,活力测定,侵袭测定和无标记成像技术。体外结果显示,MTX偶联和FTY720偶联的MSN能够有效减弱甲状腺癌细胞的增殖和侵袭,同时保持正常甲状腺细胞的脱靶效应相当低。对于更具生理相关性的体内方法,我们使用了鸡绒膜尿囊膜(CAM)分析,与接受游离药物混合物的肿瘤相比,该方法显示出甲状腺衍生异种移植物的侵袭行为减少,坏死表型增加。因此,已开发的载有多种药物的MSN可有效诱导甲状腺侵袭性癌细胞的凋亡和固定化,并有可能用作靶向药物递送的载体系统,以治疗表达叶酸受体的多种形式的侵袭性癌症。



























 京公网安备 11010802027423号
京公网安备 11010802027423号