当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Liquid Heat Capacity Measurements of the Linear Dicarboxylic Acid Family via Modulated Differential Scanning Calorimetry
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jced.9b00789 Joseph C. Bloxham 1 , Daniel Hill 1 , Thomas A. Knotts 1 , Neil F. Giles 1 , W. Vincent Wilding 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jced.9b00789 Joseph C. Bloxham 1 , Daniel Hill 1 , Thomas A. Knotts 1 , Neil F. Giles 1 , W. Vincent Wilding 1
Affiliation
|
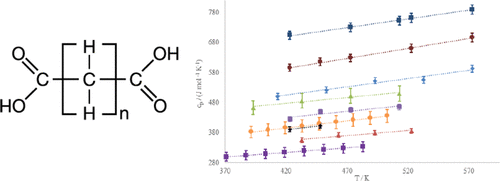
This paper reports liquid heat capacity data on members of the linear saturated dicarboxylic acid family and one dicarboxylic acid derivative measured using modulated differential scanning calorimetry. The dicarboxylic acids range in carbon number from 4 to 14. The compounds studied are dimethyl oxalate (CAS RN 553-90-2), adipic acid (1,6-hexanedioic acid, CAS RN 124-04-9), pimelic acid (1,7-heptanedioic acid, CAS RN 111-16-0), suberic acid (1,8-octanedioic acid, CAS RN 505-48-6), azelaic acid (1,9-nonanedioic acid, CAS RN 123-99-9), sebacic acid (1,10-decanedioic acid, CAS RN 111-20-6), dodecanedioic acid (1,12-dodecanedioc acid, CAS RN 693-23-2), and tetradecanedioic acid (1,14-tetradecandioic acid, CAS RN 821-38-5). The experimental results show a consistent family trend and are compared to prediction methods and data from other chemical families. A discussion of the differences in liquid heat capacity between carboxylic acids, n-alkanes, and dicarboxylic acids is presented, the accuracy of prediction through thermodynamic equations is analyzed for the family, and a correction factor for the Ruzicka–Domalski prediction method (Růžička, V. R., Jr.; Domalski, E. S. Estimation of the Heat Capacities of Organic Liquids as a Function of Temperature using Group Additivity. II. Compounds of Carbon, Hydrogen, Halogens, Nitrogen, Oxygen, and Sulfur. J. Phys. Chem. Ref. Data1993,22, 619–657.) for dicarboxylic acids is given.
中文翻译:

通过调制差示扫描量热法测量线性二羧酸家族的液体热容
本文报道了使用调制差示扫描量热法测量的线性饱和二羧酸家族成员和一种二羧酸衍生物的液体热容数据。二元羧酸的碳原子数范围为4至14。研究的化合物为草酸二甲酯(CAS RN 553-90-2),己二酸(1,6-己二酸,CAS RN 124-04-9),庚二酸(CAS 1,7-庚二酸,CAS RN 111-16-0),辛二酸(1,8-辛二酸,CAS RN 505-48-6),壬二酸(1,9-壬二酸,CAS RN 123-99) -9),癸二酸(1,10-癸二酸,CAS RN 111-20-6),十二烷二酸(1,12-十二烷二酸,CAS RN 693-23-2)和十四烷二酸(1,14-十四烷二酸,CAS RN 821-38-5)。实验结果显示出一致的家族趋势,并与其他化学家族的预测方法和数据进行了比较。讨论了羧酸之间液体热容量的差异,介绍了正构烷烃和二元羧酸,分析了通过热力学方程式预测的准确性,并针对Ruzicka–Domalski预测方法(Růžička,VR,Jr。; Domalski,ES估算热容量)进行了校正。的有机液体作为使用组可加性。II温度的函数。碳,氢,卤素,氮,氧和硫。化合物J.物理学。化学式参考文献数据1993,22,对于二羧酸619-657。)给出。
更新日期:2020-01-07
中文翻译:

通过调制差示扫描量热法测量线性二羧酸家族的液体热容
本文报道了使用调制差示扫描量热法测量的线性饱和二羧酸家族成员和一种二羧酸衍生物的液体热容数据。二元羧酸的碳原子数范围为4至14。研究的化合物为草酸二甲酯(CAS RN 553-90-2),己二酸(1,6-己二酸,CAS RN 124-04-9),庚二酸(CAS 1,7-庚二酸,CAS RN 111-16-0),辛二酸(1,8-辛二酸,CAS RN 505-48-6),壬二酸(1,9-壬二酸,CAS RN 123-99) -9),癸二酸(1,10-癸二酸,CAS RN 111-20-6),十二烷二酸(1,12-十二烷二酸,CAS RN 693-23-2)和十四烷二酸(1,14-十四烷二酸,CAS RN 821-38-5)。实验结果显示出一致的家族趋势,并与其他化学家族的预测方法和数据进行了比较。讨论了羧酸之间液体热容量的差异,介绍了正构烷烃和二元羧酸,分析了通过热力学方程式预测的准确性,并针对Ruzicka–Domalski预测方法(Růžička,VR,Jr。; Domalski,ES估算热容量)进行了校正。的有机液体作为使用组可加性。II温度的函数。碳,氢,卤素,氮,氧和硫。化合物J.物理学。化学式参考文献数据1993,22,对于二羧酸619-657。)给出。


























 京公网安备 11010802027423号
京公网安备 11010802027423号