当前位置:
X-MOL 学术
›
Chem. Geol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dual nitrogen-oxygen isotopic analysis and kinetic model for enzymatic nitrate reduction coupled with Fe(II) oxidation by Pseudogulbenkiania sp. strain 2002
Chemical Geology ( IF 3.9 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.chemgeo.2019.119456 Guojun Chen , Dandan Chen , Fangbai Li , Tongxu Liu , Zhuyu Zhao , Fang Cao
Chemical Geology ( IF 3.9 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.chemgeo.2019.119456 Guojun Chen , Dandan Chen , Fangbai Li , Tongxu Liu , Zhuyu Zhao , Fang Cao
|
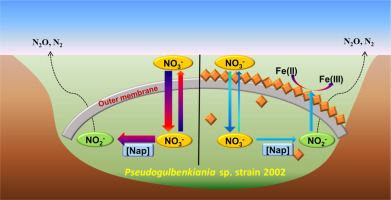
Abstract While microbially mediated coupling process of nitrate reduction and Fe(II) oxidation (NRFO) plays important role in widespread nitrogen and iron biogeochemical cycles in the natural environments, the underlying mechanism of NRFO is still not clearly understood. Here, N and O isotope fractionation during microbial nitrate reduction by Pseudogulbenkiania sp. strain 2002 with or without Fe(II) were investigated. The nitrate reduction rate decreased substantially in the presence of Fe(II), and cells became encrusted with Fe(III) minerals. However, the N isotope enrichment factors (e) with or without Fe(II) were identical within uncertainty (24.1 ± 2.4‰ and 25.1 ± 2.4‰), implying that Fe(II) oxidation had little influence on the extent of N isotope fractionation and that cell encrustation was unlikely to cause additional mass transfer limitation. Isotopic composition ratios of 18O to 15N (18e: 15e) were 0.73 ± 0.13 with and 0.50 ± 0.08 without Fe(II), close to reported values for nitrate reduction by periplasmic nitrate reductases, implying that enzymatic nitrate reduction mainly occurs in strain 2002's periplasmic space. A kinetic model based on elementary reactions was established, and rate constants of enzymatic nitrate reducing reactions were obtained in a living cell system. Combining dual N-O isotope fractionation analysis with kinetic modeling is a promising approach for interpreting diverse enzymatic pathways during NRFO processes.
中文翻译:

Pseudogulbenkiania sp. 酶促硝酸盐还原与 Fe(II) 氧化的双氮氧同位素分析和动力学模型。应变 2002
摘要 虽然微生物介导的硝酸盐还原和 Fe(II) 氧化 (NRFO) 的耦合过程在自然环境中广泛的氮和铁生物地球化学循环中起着重要作用,但 NRFO 的潜在机制仍不清楚。在这里,Pseudogulbenkiania sp. 在微生物硝酸盐还原过程中的 N 和 O 同位素分馏。研究了含有或不含 Fe(II) 的菌株 2002。在 Fe(II) 存在下,硝酸盐还原率显着降低,细胞被 Fe(III) 矿物质包裹。然而,含或不含 Fe(II) 的 N 同位素富集因子 (e) 在不确定度 (24.1 ± 2.4‰ 和 25.1 ± 2.4‰) 内是相同的,这意味着 Fe(II) 氧化对 N 同位素分馏的程度几乎没有影响,并且细胞结壳不太可能导致额外的传质限制。18O 与 15N (18e: 15e) 的同位素组成比为 0.73 ± 0.13 和 0.50 ± 0.08 没有 Fe(II),接近报道的周质硝酸盐还原酶还原硝酸盐的值,这意味着酶促硝酸盐还原主要发生在菌株 2002 的周质中空间。建立了基于基元反应的动力学模型,获得了活细胞系统中酶促硝酸盐还原反应的速率常数。将双 NO 同位素分馏分析与动力学建模相结合,是解释 NRFO 过程中不同酶途径的一种很有前景的方法。13 和 0.50 ± 0.08 没有 Fe(II),接近报道的周质硝酸盐还原酶还原硝酸盐的值,这意味着酶促硝酸盐还原主要发生在菌株 2002 的周质空间。建立了基于基元反应的动力学模型,获得了活细胞系统中酶促硝酸盐还原反应的速率常数。将双 NO 同位素分馏分析与动力学建模相结合,是解释 NRFO 过程中不同酶途径的一种很有前景的方法。13 和 0.50 ± 0.08 没有 Fe(II),接近报道的周质硝酸盐还原酶还原硝酸盐的值,这意味着酶促硝酸盐还原主要发生在菌株 2002 的周质空间。建立了基于基元反应的动力学模型,获得了活细胞系统中酶促硝酸盐还原反应的速率常数。将双 NO 同位素分馏分析与动力学模型相结合,是解释 NRFO 过程中不同酶途径的一种很有前景的方法。在活细胞系统中获得了酶促硝酸盐还原反应的速率常数和速率常数。将双 NO 同位素分馏分析与动力学建模相结合,是解释 NRFO 过程中不同酶途径的一种很有前景的方法。在活细胞系统中获得了酶促硝酸盐还原反应的速率常数和速率常数。将双 NO 同位素分馏分析与动力学建模相结合,是解释 NRFO 过程中不同酶途径的一种很有前景的方法。
更新日期:2020-02-01
中文翻译:

Pseudogulbenkiania sp. 酶促硝酸盐还原与 Fe(II) 氧化的双氮氧同位素分析和动力学模型。应变 2002
摘要 虽然微生物介导的硝酸盐还原和 Fe(II) 氧化 (NRFO) 的耦合过程在自然环境中广泛的氮和铁生物地球化学循环中起着重要作用,但 NRFO 的潜在机制仍不清楚。在这里,Pseudogulbenkiania sp. 在微生物硝酸盐还原过程中的 N 和 O 同位素分馏。研究了含有或不含 Fe(II) 的菌株 2002。在 Fe(II) 存在下,硝酸盐还原率显着降低,细胞被 Fe(III) 矿物质包裹。然而,含或不含 Fe(II) 的 N 同位素富集因子 (e) 在不确定度 (24.1 ± 2.4‰ 和 25.1 ± 2.4‰) 内是相同的,这意味着 Fe(II) 氧化对 N 同位素分馏的程度几乎没有影响,并且细胞结壳不太可能导致额外的传质限制。18O 与 15N (18e: 15e) 的同位素组成比为 0.73 ± 0.13 和 0.50 ± 0.08 没有 Fe(II),接近报道的周质硝酸盐还原酶还原硝酸盐的值,这意味着酶促硝酸盐还原主要发生在菌株 2002 的周质中空间。建立了基于基元反应的动力学模型,获得了活细胞系统中酶促硝酸盐还原反应的速率常数。将双 NO 同位素分馏分析与动力学建模相结合,是解释 NRFO 过程中不同酶途径的一种很有前景的方法。13 和 0.50 ± 0.08 没有 Fe(II),接近报道的周质硝酸盐还原酶还原硝酸盐的值,这意味着酶促硝酸盐还原主要发生在菌株 2002 的周质空间。建立了基于基元反应的动力学模型,获得了活细胞系统中酶促硝酸盐还原反应的速率常数。将双 NO 同位素分馏分析与动力学建模相结合,是解释 NRFO 过程中不同酶途径的一种很有前景的方法。13 和 0.50 ± 0.08 没有 Fe(II),接近报道的周质硝酸盐还原酶还原硝酸盐的值,这意味着酶促硝酸盐还原主要发生在菌株 2002 的周质空间。建立了基于基元反应的动力学模型,获得了活细胞系统中酶促硝酸盐还原反应的速率常数。将双 NO 同位素分馏分析与动力学模型相结合,是解释 NRFO 过程中不同酶途径的一种很有前景的方法。在活细胞系统中获得了酶促硝酸盐还原反应的速率常数和速率常数。将双 NO 同位素分馏分析与动力学建模相结合,是解释 NRFO 过程中不同酶途径的一种很有前景的方法。在活细胞系统中获得了酶促硝酸盐还原反应的速率常数和速率常数。将双 NO 同位素分馏分析与动力学建模相结合,是解释 NRFO 过程中不同酶途径的一种很有前景的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号