当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cytotoxic and non-cytotoxic cardiac glycosides isolated from the combined flowers, leaves, and twigs of Streblus asper.
Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.bmc.2019.115301 Yulin Ren 1 , Qingwei Tan 1 , Kimberly Heath 2 , Sijin Wu 1 , James R Wilson 1 , Jinhong Ren 3 , Pratik Shriwas 4 , Chunhua Yuan 5 , Tran Ngoc Ninh 6 , Hee-Byung Chai 1 , Xiaozhuo Chen 7 , Djaja D Soejarto 8 , Michael E Johnson 9 , Xiaolin Cheng 1 , Joanna E Burdette 2 , A Douglas Kinghorn 1
Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.bmc.2019.115301 Yulin Ren 1 , Qingwei Tan 1 , Kimberly Heath 2 , Sijin Wu 1 , James R Wilson 1 , Jinhong Ren 3 , Pratik Shriwas 4 , Chunhua Yuan 5 , Tran Ngoc Ninh 6 , Hee-Byung Chai 1 , Xiaozhuo Chen 7 , Djaja D Soejarto 8 , Michael E Johnson 9 , Xiaolin Cheng 1 , Joanna E Burdette 2 , A Douglas Kinghorn 1
Affiliation
|
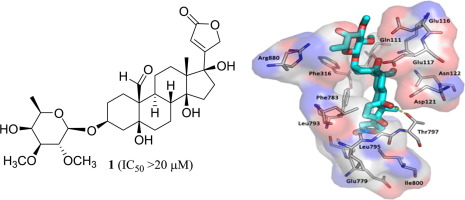
A new non-cytotoxic [(+)-17β-hydroxystrebloside (1)] and two known cytotoxic [(+)-3'-de-O-methylkamaloside (2) and (+)-strebloside (3)] cardiac glycosides were isolated and identified from the combined flowers, leaves, and twigs of Streblus asper collected in Vietnam, with the absolute configuration of 1 established from analysis of its ECD and NMR spectroscopic data and confirmed by computational ECD calculations. A new 14,21-epoxycardanolide (3a) was synthesized from 3 that was treated with base. A preliminary structure-activity relationship study indicated that the C-14 hydroxy group and the C-17 lactone unit and the established conformation are important for the mediation of the cytotoxicity of 3. Molecular docking profiles showed that the cytotoxic 3 and its non-cytotoxic analogue 1 bind differentially to Na+/K+-ATPase. Compound 3 docks deeply in the Na+/K+-ATPase pocket with a sole pose, and its C-10 formyl and C-5, C-14, and C-4' hydroxy groups may form hydrogen bonds with the side-chains of Glu111, Glu117, Thr797, and Arg880 of Na+/K+-ATPase, respectively. However, 1 fits the cation binding sites with at least three different poses, which all depotentiate the binding between 1 and Na+/K+-ATPase. Thus, 3 was found to inhibit Na+/K+-ATPase, but 1 did not. In addition, the cytotoxic and Na+/K+-ATPase inhibitory 3 did not affect glucose uptake in human lung cancer cells, against which it showed potent activity, indicating that this cardiac glycoside mediates its cytotoxicity by targeting Na+/K+-ATPase but not by interacting with glucose transporters.
中文翻译:

从Streblus asper的花朵,叶子和细枝中分离得到的细胞毒性和非细胞毒性强心苷。
一种新的无细胞毒性的[(+)-17β-羟基硬脂糖苷(1)]和两种已知的细胞毒性的[(+)-3'-de-O-甲基kamaloside(2)和(+)-硬脂苷(3)]强心苷是从越南收集的Streblus asper的花朵,叶子和细枝中分离和鉴定,其绝对构型1通过对其ECD和NMR光谱数据的分析确定,并通过计算ECD计算得到确认。由碱处理的3合成了新的14,21-环氧腰果酚(3a)。初步的结构-活性关系研究表明,C-14羟基和C-17内酯单元及已确定的构象对于介导3的细胞毒性很重要。分子对接分析表明,细胞毒性3及其非细胞毒性类似物1与Na + / K + -ATP酶差异结合。化合物3独自摆在Na + / K + -ATPase口袋深处,其C-10甲酰基和C-5,C-14和C-4'羟基可与Glu111的侧链形成氢键,Na + / K + -ATPase的Glu117,Thr797和Arg880。然而,1使阳离子结合位点具有至少三个不同的姿势,它们都使1和Na + / K + -ATPase之间的结合减弱。因此,发现3种抑制Na + / K + -ATP酶,但1种没有抑制。此外,细胞毒性和Na + / K + -ATPase抑制因子3不会影响人肺癌细胞的葡萄糖摄取,对它具有强大的活性,表明该强心苷通过靶向Na + / K + -ATPase而不是通过相互作用来介导其细胞毒性。与葡萄糖转运蛋白。羟基可分别与Na + / K + -ATPase的Glu111,Glu117,Thr797和Arg880的侧链形成氢键。然而,1使阳离子结合位点具有至少三个不同的姿势,它们都使1和Na + / K + -ATPase之间的结合减弱。因此,发现3种抑制Na + / K + -ATP酶,但1种没有抑制。此外,细胞毒性和Na + / K + -ATPase抑制因子3不会影响人肺癌细胞的葡萄糖摄取,对它具有强大的活性,表明该强心苷通过靶向Na + / K + -ATPase而不是通过相互作用来介导其细胞毒性。与葡萄糖转运蛋白。羟基可分别与Na + / K + -ATPase的Glu111,Glu117,Thr797和Arg880的侧链形成氢键。然而,1使阳离子结合位点具有至少三个不同的姿势,它们都使1和Na + / K + -ATPase之间的结合减弱。因此,发现3种抑制Na + / K + -ATP酶,但1种没有抑制。此外,细胞毒性和Na + / K + -ATPase抑制因子3不会影响人肺癌细胞的葡萄糖摄取,对它具有强大的活性,表明该强心苷通过靶向Na + / K + -ATPase而不是通过相互作用来介导其细胞毒性。与葡萄糖转运蛋白。这些都使1和Na + / K + -ATPase之间的结合减弱。因此,发现3种抑制Na + / K + -ATP酶,但1种没有抑制。此外,细胞毒性和Na + / K + -ATPase抑制因子3不会影响人肺癌细胞的葡萄糖摄取,对它具有强大的活性,表明该强心苷通过靶向Na + / K + -ATPase而不是通过相互作用来介导其细胞毒性。与葡萄糖转运蛋白。这些都使1和Na + / K + -ATPase之间的结合减弱。因此,发现3种抑制Na + / K + -ATP酶,但1种没有抑制。此外,细胞毒性和Na + / K + -ATPase抑制因子3不会影响人肺癌细胞的葡萄糖摄取,对它具有强大的活性,表明该强心苷通过靶向Na + / K + -ATPase而不是通过相互作用来介导其细胞毒性。与葡萄糖转运蛋白。
更新日期:2020-01-07
中文翻译:

从Streblus asper的花朵,叶子和细枝中分离得到的细胞毒性和非细胞毒性强心苷。
一种新的无细胞毒性的[(+)-17β-羟基硬脂糖苷(1)]和两种已知的细胞毒性的[(+)-3'-de-O-甲基kamaloside(2)和(+)-硬脂苷(3)]强心苷是从越南收集的Streblus asper的花朵,叶子和细枝中分离和鉴定,其绝对构型1通过对其ECD和NMR光谱数据的分析确定,并通过计算ECD计算得到确认。由碱处理的3合成了新的14,21-环氧腰果酚(3a)。初步的结构-活性关系研究表明,C-14羟基和C-17内酯单元及已确定的构象对于介导3的细胞毒性很重要。分子对接分析表明,细胞毒性3及其非细胞毒性类似物1与Na + / K + -ATP酶差异结合。化合物3独自摆在Na + / K + -ATPase口袋深处,其C-10甲酰基和C-5,C-14和C-4'羟基可与Glu111的侧链形成氢键,Na + / K + -ATPase的Glu117,Thr797和Arg880。然而,1使阳离子结合位点具有至少三个不同的姿势,它们都使1和Na + / K + -ATPase之间的结合减弱。因此,发现3种抑制Na + / K + -ATP酶,但1种没有抑制。此外,细胞毒性和Na + / K + -ATPase抑制因子3不会影响人肺癌细胞的葡萄糖摄取,对它具有强大的活性,表明该强心苷通过靶向Na + / K + -ATPase而不是通过相互作用来介导其细胞毒性。与葡萄糖转运蛋白。羟基可分别与Na + / K + -ATPase的Glu111,Glu117,Thr797和Arg880的侧链形成氢键。然而,1使阳离子结合位点具有至少三个不同的姿势,它们都使1和Na + / K + -ATPase之间的结合减弱。因此,发现3种抑制Na + / K + -ATP酶,但1种没有抑制。此外,细胞毒性和Na + / K + -ATPase抑制因子3不会影响人肺癌细胞的葡萄糖摄取,对它具有强大的活性,表明该强心苷通过靶向Na + / K + -ATPase而不是通过相互作用来介导其细胞毒性。与葡萄糖转运蛋白。羟基可分别与Na + / K + -ATPase的Glu111,Glu117,Thr797和Arg880的侧链形成氢键。然而,1使阳离子结合位点具有至少三个不同的姿势,它们都使1和Na + / K + -ATPase之间的结合减弱。因此,发现3种抑制Na + / K + -ATP酶,但1种没有抑制。此外,细胞毒性和Na + / K + -ATPase抑制因子3不会影响人肺癌细胞的葡萄糖摄取,对它具有强大的活性,表明该强心苷通过靶向Na + / K + -ATPase而不是通过相互作用来介导其细胞毒性。与葡萄糖转运蛋白。这些都使1和Na + / K + -ATPase之间的结合减弱。因此,发现3种抑制Na + / K + -ATP酶,但1种没有抑制。此外,细胞毒性和Na + / K + -ATPase抑制因子3不会影响人肺癌细胞的葡萄糖摄取,对它具有强大的活性,表明该强心苷通过靶向Na + / K + -ATPase而不是通过相互作用来介导其细胞毒性。与葡萄糖转运蛋白。这些都使1和Na + / K + -ATPase之间的结合减弱。因此,发现3种抑制Na + / K + -ATP酶,但1种没有抑制。此外,细胞毒性和Na + / K + -ATPase抑制因子3不会影响人肺癌细胞的葡萄糖摄取,对它具有强大的活性,表明该强心苷通过靶向Na + / K + -ATPase而不是通过相互作用来介导其细胞毒性。与葡萄糖转运蛋白。


























 京公网安备 11010802027423号
京公网安备 11010802027423号