当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemically Enabled Intramolecular Aminooxygenation of Alkynes via Amidyl Radical Cyclization
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-02-21 , DOI: 10.1002/cjoc.201900500 Zhong‐Wei Hou 1 , Hai‐Chao Xu 1
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-02-21 , DOI: 10.1002/cjoc.201900500 Zhong‐Wei Hou 1 , Hai‐Chao Xu 1
Affiliation
|
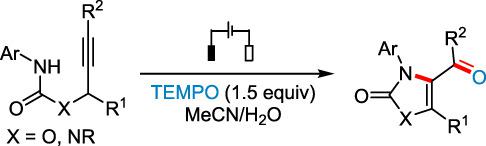
An electrochemical synthesis of oxazol‐2‐ones and imidazol‐2‐ones has been developed via 5‐exo‐dig cyclization of propargylic carbamates‐ and ureas‐derived amidyl radicals. The electrosynthesis relies on the dual function of 2,2,6,6‐tetramethylpiperidin‐ 1‐yl)oxyl (TEMPO) as a redox mediator for amidyl radical formation and an oxygen atom donor. The reactions are conducted under mild conditions using a simple setup and provide convenient access to functionalized oxazol‐2‐ones and imidazol‐2‐ones from readily available materials.
中文翻译:

通过酰胺基自由基环化实现炔烃的电化学分子内氨基氧化
恶唑-2-酮和咪唑-2-酮的电化学合成已经开发经由-5-外-挖炔carbamates-和环化的脲衍生的酰胺基团。电合成依赖于2,2,6,6-四甲基哌啶-1-基)氧基(TEMPO)的双重功能,作为氧化还原介质,用于形成酰胺基和氧原子供体。该反应可在温和条件下使用简单的设置进行,并可以方便地从容易获得的材料中获得官能化的恶唑-2-酮和咪唑-2-酮。
更新日期:2020-02-21
中文翻译:

通过酰胺基自由基环化实现炔烃的电化学分子内氨基氧化
恶唑-2-酮和咪唑-2-酮的电化学合成已经开发经由-5-外-挖炔carbamates-和环化的脲衍生的酰胺基团。电合成依赖于2,2,6,6-四甲基哌啶-1-基)氧基(TEMPO)的双重功能,作为氧化还原介质,用于形成酰胺基和氧原子供体。该反应可在温和条件下使用简单的设置进行,并可以方便地从容易获得的材料中获得官能化的恶唑-2-酮和咪唑-2-酮。


























 京公网安备 11010802027423号
京公网安备 11010802027423号