当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stepwise Mechanism for the Bromination of Arenes by a Hypervalent Iodine Reagent.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.joc.9b02784 Albert Granados 1 , Alexandr Shafir 2 , Ana Arrieta 3 , Fernando P Cossío 3 , Adelina Vallribera 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.joc.9b02784 Albert Granados 1 , Alexandr Shafir 2 , Ana Arrieta 3 , Fernando P Cossío 3 , Adelina Vallribera 1
Affiliation
|
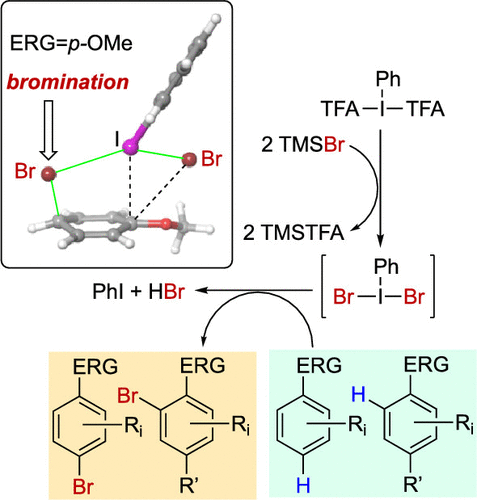
A mild, metal-free bromination method of arenes has been developed using the combination of bis(trifluoroacetoxy)iodobencene and trimethylsilyl bromide. In situ-formed dibromo(phenyl)-λ3-iodane (PhIBr2) is proposed as the reactive intermediate. This methodology using PIFA/TMSBr has been applied with success to a great number of substrates (25 examples). The treatment of mono-substituted activated arenes led to para-brominated products (2u-z) in excellent 83-96% yields. Density functional theory calculations indicate a stepwise mechanism involving a double bromine addition followed by a type II dyotropic reaction with concomitant re-aromatization of the six-membered ring.
中文翻译:

高价碘试剂溴化芳烃的逐步机理。
使用双(三氟乙酰氧基)碘苯苄酯和三甲基甲硅烷基溴化物的组合,开发了一种温和,无金属的芳烃溴化方法。提出了原位形成的二溴(苯基)-λ3-碘(PhIBr2)作为反应中间体。使用PIFA / TMSBr的这种方法已成功应用于大量基材(25个实例)。单取代的活化芳烃的处理产生对溴化产物(2u-z),产率高达83-96%。密度泛函理论计算表明了一种逐步机理,该机理涉及添加双溴,然后进行II型正离子反应,并伴随六元环的重新芳构化。
更新日期:2020-01-17
中文翻译:

高价碘试剂溴化芳烃的逐步机理。
使用双(三氟乙酰氧基)碘苯苄酯和三甲基甲硅烷基溴化物的组合,开发了一种温和,无金属的芳烃溴化方法。提出了原位形成的二溴(苯基)-λ3-碘(PhIBr2)作为反应中间体。使用PIFA / TMSBr的这种方法已成功应用于大量基材(25个实例)。单取代的活化芳烃的处理产生对溴化产物(2u-z),产率高达83-96%。密度泛函理论计算表明了一种逐步机理,该机理涉及添加双溴,然后进行II型正离子反应,并伴随六元环的重新芳构化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号