当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deoxygenative Amination of Azine-N-oxides with Acyl Azides via [3 + 2] Cycloaddition.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.joc.9b03173 Dongeun Kim 1 , Prithwish Ghosh 1 , Na Yeon Kwon 1 , Sang Hoon Han 1 , Sangil Han 1 , Neeraj Kumar Mishra 1 , Saegun Kim 1 , In Su Kim 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.joc.9b03173 Dongeun Kim 1 , Prithwish Ghosh 1 , Na Yeon Kwon 1 , Sang Hoon Han 1 , Sangil Han 1 , Neeraj Kumar Mishra 1 , Saegun Kim 1 , In Su Kim 1
Affiliation
|
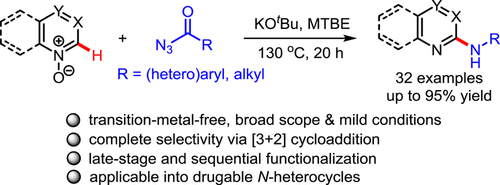
A transition-metal-free deoxygenative C-H amination reaction of azine-N-oxides with acyl azides is described. The initial formation of an isocyanate from the starting acyl azide via a Curtius rearrangement can trigger a [3 + 2] dipolar cycloaddition of polar N-oxide fragments to generate the aminated azine derivative. The applicability of this method is highlighted by the late-stage and sequential amination reactions of complex bioactive compounds, including quinidine and fasudil. Moreover, the direct transformation of aminated azines into various bioactive N-heterocycles illustrates the significance of this newly developed protocol.
中文翻译:

通过[3 + 2]环加成反应,用酰基叠氮化物对叠氮-N-氧化物进行脱氧胺化。
描述了一种无过渡金属的嗪-N-氧化物与酰基叠氮化物的脱氧CH胺化反应。通过库尔修斯重排从起始酰基叠氮化物初步形成异氰酸酯可以触发极性N-氧化物片段的[3 + 2]双极性环加成反应,从而生成胺化的嗪衍生物。复杂的生物活性化合物(包括奎尼丁和法舒地尔)的后期和顺序胺化反应突出了该方法的适用性。此外,将胺化嗪直接转化为各种生物活性的N-杂环说明了这种新开发的方案的重要性。
更新日期:2020-01-17
中文翻译:

通过[3 + 2]环加成反应,用酰基叠氮化物对叠氮-N-氧化物进行脱氧胺化。
描述了一种无过渡金属的嗪-N-氧化物与酰基叠氮化物的脱氧CH胺化反应。通过库尔修斯重排从起始酰基叠氮化物初步形成异氰酸酯可以触发极性N-氧化物片段的[3 + 2]双极性环加成反应,从而生成胺化的嗪衍生物。复杂的生物活性化合物(包括奎尼丁和法舒地尔)的后期和顺序胺化反应突出了该方法的适用性。此外,将胺化嗪直接转化为各种生物活性的N-杂环说明了这种新开发的方案的重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号