当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Untargeted Proteomics-Based Profiling for the Identification of Novel Processing-Induced Protein Modifications in Milk.
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2020-01-29 , DOI: 10.1021/acs.jproteome.9b00630 Jasmin Meltretter 1 , Johannes Wüst 1 , Daniel Dittrich 1 , Johannes Lach 2 , Jonas Ludwig 2 , Jutta Eichler 2 , Monika Pischetsrieder 1
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2020-01-29 , DOI: 10.1021/acs.jproteome.9b00630 Jasmin Meltretter 1 , Johannes Wüst 1 , Daniel Dittrich 1 , Johannes Lach 2 , Jonas Ludwig 2 , Jutta Eichler 2 , Monika Pischetsrieder 1
Affiliation
|
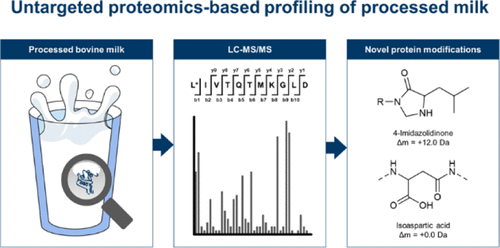
Nonenzymatic post-translational protein modifications (nePTMs) affect the nutritional, physiological, and technological properties of proteins in food and in vivo. In contrast to the usual targeted analyses, the present study determined nePTMs in processed milk in a truly untargeted proteomic approach. Thus, it was possible to determine to which extent known nePTM structures explain protein modifications in processed milk and to detect and identify novel products. The method combined ultrahigh-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry with bioinformatic data analysis by the software XCMS. The nePTMs detected by untargeted profiling of a β-lactoglobulin-lactose model were incorporated in a sensitive scheduled multiple reaction monitoring method to analyze these modifications in milk samples and to monitor their reaction kinetics during thermal treatment. Additionally, we identified the structures of unknown modifications. Lactosylation, carboxymethylation, formylation of lysine and N-terminus, glycation of arginine, oxidation of methionine, tryptophan, and cysteine, oxidative deamination of N-terminus, and deamidation of asparagine and glutamine were the most important reactions of β-lactoglobulin during milk processing. The isomerization of aspartic acid was observed for the first time in milk products, and N-terminal 4-imidazolidinone was identified as a novel nePTM.
中文翻译:

基于目标蛋白质组学的分析,用于鉴定牛奶中新型加工诱导的蛋白质修饰。
非酶翻译后蛋白质修饰(nePTM)影响食物和体内蛋白质的营养,生理和技术特性。与通常的靶向分析相反,本研究以真正非靶向的蛋白质组学方法确定了加工乳中的nePTM。因此,可以确定已知的nePTM结构在多大程度上解释了加工乳中的蛋白质修饰,并可以检测和鉴定新产品。该方法将超高效液相色谱与电喷雾电离串联质谱联用,并通过XCMS软件进行生物信息学数据分析。通过对β-乳球蛋白-乳糖模型进行非靶向分析而检测到的nePTM被纳入了灵敏的计划多重反应监测方法中,以分析牛奶样品中的这些修饰并监测热处理过程中的反应动力学。此外,我们确定了未知修饰的结构。乳加工过程中,β-乳球蛋白最重要的反应是乳化,羧甲基化,赖氨酸和N末端的甲酰化,精氨酸的糖基化,蛋氨酸,色氨酸和半胱氨酸的氧化,N末端的氧化脱氨基以及天冬酰胺和谷氨酰胺的脱酰胺化。 。首次在乳制品中观察到天冬氨酸的异构化,N末端的4-咪唑啉酮被鉴定为新型的nePTM。此外,我们确定了未知修饰的结构。乳加工过程中,β-乳球蛋白最重要的反应是乳化,羧甲基化,赖氨酸和N末端的甲酰化,精氨酸的糖基化,蛋氨酸,色氨酸和半胱氨酸的氧化,N末端的氧化脱氨基以及天冬酰胺和谷氨酰胺的脱酰胺化。 。在乳制品中首次观察到天冬氨酸的异构化,N末端的4-咪唑啉酮被鉴定为新型的nePTM。此外,我们确定了未知修饰的结构。乳加工过程中,β-乳球蛋白最重要的反应是乳化,羧甲基化,赖氨酸和N末端的甲酰化,精氨酸的糖基化,蛋氨酸,色氨酸和半胱氨酸的氧化,N末端的氧化脱氨基以及天冬酰胺和谷氨酰胺的脱酰胺化。 。在乳制品中首次观察到天冬氨酸的异构化,N末端的4-咪唑啉酮被鉴定为新型的nePTM。乳加工过程中,β-乳球蛋白的最重要反应是天冬酰胺和谷氨酰胺的脱酰胺和酰胺化。在乳制品中首次观察到天冬氨酸的异构化,N末端的4-咪唑啉酮被鉴定为新型的nePTM。乳加工过程中,β-乳球蛋白的最重要反应是天冬酰胺和谷氨酰胺的脱酰胺和酰胺化。在乳制品中首次观察到天冬氨酸的异构化,N末端的4-咪唑啉酮被鉴定为新型的nePTM。
更新日期:2020-01-29
中文翻译:

基于目标蛋白质组学的分析,用于鉴定牛奶中新型加工诱导的蛋白质修饰。
非酶翻译后蛋白质修饰(nePTM)影响食物和体内蛋白质的营养,生理和技术特性。与通常的靶向分析相反,本研究以真正非靶向的蛋白质组学方法确定了加工乳中的nePTM。因此,可以确定已知的nePTM结构在多大程度上解释了加工乳中的蛋白质修饰,并可以检测和鉴定新产品。该方法将超高效液相色谱与电喷雾电离串联质谱联用,并通过XCMS软件进行生物信息学数据分析。通过对β-乳球蛋白-乳糖模型进行非靶向分析而检测到的nePTM被纳入了灵敏的计划多重反应监测方法中,以分析牛奶样品中的这些修饰并监测热处理过程中的反应动力学。此外,我们确定了未知修饰的结构。乳加工过程中,β-乳球蛋白最重要的反应是乳化,羧甲基化,赖氨酸和N末端的甲酰化,精氨酸的糖基化,蛋氨酸,色氨酸和半胱氨酸的氧化,N末端的氧化脱氨基以及天冬酰胺和谷氨酰胺的脱酰胺化。 。首次在乳制品中观察到天冬氨酸的异构化,N末端的4-咪唑啉酮被鉴定为新型的nePTM。此外,我们确定了未知修饰的结构。乳加工过程中,β-乳球蛋白最重要的反应是乳化,羧甲基化,赖氨酸和N末端的甲酰化,精氨酸的糖基化,蛋氨酸,色氨酸和半胱氨酸的氧化,N末端的氧化脱氨基以及天冬酰胺和谷氨酰胺的脱酰胺化。 。在乳制品中首次观察到天冬氨酸的异构化,N末端的4-咪唑啉酮被鉴定为新型的nePTM。此外,我们确定了未知修饰的结构。乳加工过程中,β-乳球蛋白最重要的反应是乳化,羧甲基化,赖氨酸和N末端的甲酰化,精氨酸的糖基化,蛋氨酸,色氨酸和半胱氨酸的氧化,N末端的氧化脱氨基以及天冬酰胺和谷氨酰胺的脱酰胺化。 。在乳制品中首次观察到天冬氨酸的异构化,N末端的4-咪唑啉酮被鉴定为新型的nePTM。乳加工过程中,β-乳球蛋白的最重要反应是天冬酰胺和谷氨酰胺的脱酰胺和酰胺化。在乳制品中首次观察到天冬氨酸的异构化,N末端的4-咪唑啉酮被鉴定为新型的nePTM。乳加工过程中,β-乳球蛋白的最重要反应是天冬酰胺和谷氨酰胺的脱酰胺和酰胺化。在乳制品中首次观察到天冬氨酸的异构化,N末端的4-咪唑啉酮被鉴定为新型的nePTM。



























 京公网安备 11010802027423号
京公网安备 11010802027423号