当前位置:
X-MOL 学术
›
Genet. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Higher dosing of alglucosidase alfa improves outcomes in children with Pompe disease: a clinical study and review of the literature.
Genetics in Medicine ( IF 8.8 ) Pub Date : 2020-01-06 , DOI: 10.1038/s41436-019-0738-0 Aleena A Khan 1 , Laura E Case 2 , Mrudu Herbert 1 , Stephanie DeArmey 1 , Harrison Jones 3, 4 , Kelly Crisp 3 , Kanecia Zimmerman 5 , Mai K ElMallah 6 , Sarah P Young 1 , Priya S Kishnani 1
Genetics in Medicine ( IF 8.8 ) Pub Date : 2020-01-06 , DOI: 10.1038/s41436-019-0738-0 Aleena A Khan 1 , Laura E Case 2 , Mrudu Herbert 1 , Stephanie DeArmey 1 , Harrison Jones 3, 4 , Kelly Crisp 3 , Kanecia Zimmerman 5 , Mai K ElMallah 6 , Sarah P Young 1 , Priya S Kishnani 1
Affiliation
|
PURPOSE
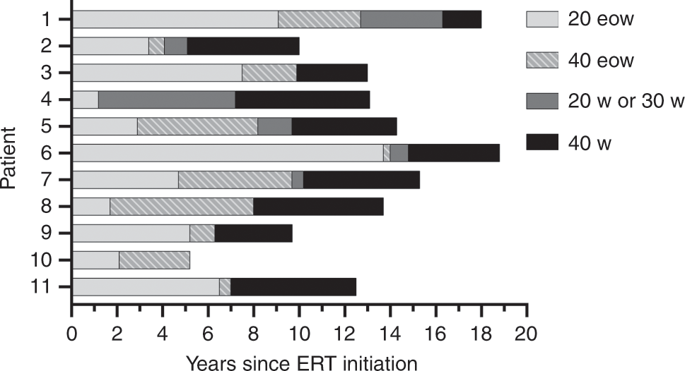
Enzyme replacement therapy (ERT) with recombinant human acid-α glucosidase (rhGAA) at standard dose of 20 mg/kg every other week is insufficient to halt the long-term progression of myopathy in Pompe disease.
METHODS
We conducted a retrospective study on infantile-onset Pompe disease (IPD) and late-onset Pompe disease (LOPD) patients with onset before age 5 years, ≥12 months of treatment with standard dose ERT, and rhGAA immunogenic tolerance prior to dose escalation. Long-term follow-up of up to 18 years was obtained. We obtained physical therapy, lingual strength, biochemical, and pulmonary assessments as dose was escalated.
RESULTS
Eleven patients with IPD (n = 7) or LOPD (n = 4) were treated with higher doses of up to 40 mg/kg weekly. There were improvements in gross motor function measure in 9/10 patients, in lingual strength in 6/6 patients, and in pulmonary function in 4/11. Significant reductions in urinary glucose tetrasaccharide, creatine kinase, aspartate aminotransferase, and alanine aminotransferase were observed at 40 mg/kg weekly compared with lower doses (p < 0.05). No safety or immunogenicity concerns were observed at higher doses.
CONCLUSION
Higher rhGAA doses are safe, improve gross motor outcomes, lingual strength, pulmonary function measures, and biochemical markers in early-onset Pompe disease, and should be considered in patients with clinical and functional decline.
中文翻译:

更高剂量的阿糖苷酶 alfa 可改善庞贝病儿童的预后:一项临床研究和文献回顾。
目的 每隔一周使用标准剂量 20 mg/kg 的重组人酸性-α 葡萄糖苷酶 (rhGAA) 进行酶替代疗法 (ERT) 不足以阻止庞贝病肌病的长期进展。方法 我们对 5 岁前发病、标准剂量 ERT 治疗≥12 个月、剂量递增前 rhGAA 免疫原性耐受的婴儿型庞贝病 (IPD) 和晚发型庞贝病 (LOPD) 患者进行了回顾性研究. 获得了长达 18 年的长期随访。随着剂量的增加,我们获得了物理治疗、舌力、生化和肺部评估。结果 11 名 IPD (n = 7) 或 LOPD (n = 4) 患者每周接受高达 40 mg/kg 的更高剂量治疗。9/10 患者的粗大运动功能测量有改善,6/6 患者的语言强度和 4/11 患者的肺功能。与较低剂量相比,每周 40 mg/kg 观察到尿葡萄糖四糖、肌酸激酶、天冬氨酸氨基转移酶和丙氨酸氨基转移酶显着降低(p < 0.05)。在较高剂量下未观察到安全性或免疫原性问题。结论 较高的 rhGAA 剂量是安全的,可改善早发性庞贝病的粗大运动结果、舌肌强度、肺功能测量和生化标志物,应考虑用于临床和功能下降的患者。在较高剂量下未观察到安全性或免疫原性问题。结论 较高的 rhGAA 剂量是安全的,可改善早发性庞贝病的粗大运动结果、舌肌强度、肺功能测量和生化标志物,应考虑用于临床和功能下降的患者。在较高剂量下未观察到安全性或免疫原性问题。结论 较高的 rhGAA 剂量是安全的,可改善早发性庞贝病的粗大运动结果、舌肌强度、肺功能测量和生化标志物,应考虑用于临床和功能下降的患者。
更新日期:2020-01-06
中文翻译:

更高剂量的阿糖苷酶 alfa 可改善庞贝病儿童的预后:一项临床研究和文献回顾。
目的 每隔一周使用标准剂量 20 mg/kg 的重组人酸性-α 葡萄糖苷酶 (rhGAA) 进行酶替代疗法 (ERT) 不足以阻止庞贝病肌病的长期进展。方法 我们对 5 岁前发病、标准剂量 ERT 治疗≥12 个月、剂量递增前 rhGAA 免疫原性耐受的婴儿型庞贝病 (IPD) 和晚发型庞贝病 (LOPD) 患者进行了回顾性研究. 获得了长达 18 年的长期随访。随着剂量的增加,我们获得了物理治疗、舌力、生化和肺部评估。结果 11 名 IPD (n = 7) 或 LOPD (n = 4) 患者每周接受高达 40 mg/kg 的更高剂量治疗。9/10 患者的粗大运动功能测量有改善,6/6 患者的语言强度和 4/11 患者的肺功能。与较低剂量相比,每周 40 mg/kg 观察到尿葡萄糖四糖、肌酸激酶、天冬氨酸氨基转移酶和丙氨酸氨基转移酶显着降低(p < 0.05)。在较高剂量下未观察到安全性或免疫原性问题。结论 较高的 rhGAA 剂量是安全的,可改善早发性庞贝病的粗大运动结果、舌肌强度、肺功能测量和生化标志物,应考虑用于临床和功能下降的患者。在较高剂量下未观察到安全性或免疫原性问题。结论 较高的 rhGAA 剂量是安全的,可改善早发性庞贝病的粗大运动结果、舌肌强度、肺功能测量和生化标志物,应考虑用于临床和功能下降的患者。在较高剂量下未观察到安全性或免疫原性问题。结论 较高的 rhGAA 剂量是安全的,可改善早发性庞贝病的粗大运动结果、舌肌强度、肺功能测量和生化标志物,应考虑用于临床和功能下降的患者。



























 京公网安备 11010802027423号
京公网安备 11010802027423号