当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bis(ethylmaltolato)oxidovanadium(iv) inhibited the pathogenesis of Alzheimer's disease in triple transgenic model mice.
Metallomics ( IF 3.4 ) Pub Date : 2020-01-23 , DOI: 10.1039/c9mt00271e Zhijun He 1 , Shuangxue Han , Chong Wu , Lina Liu , Huazhang Zhu , Ang Liu , Qiying Lu , Jingqiang Huang , Xiubo Du , Nan Li , Qinguo Xie , Lu Wan , Jiazuan Ni , Lingling Chen , Xiaogai Yang , Qiong Liu
Metallomics ( IF 3.4 ) Pub Date : 2020-01-23 , DOI: 10.1039/c9mt00271e Zhijun He 1 , Shuangxue Han , Chong Wu , Lina Liu , Huazhang Zhu , Ang Liu , Qiying Lu , Jingqiang Huang , Xiubo Du , Nan Li , Qinguo Xie , Lu Wan , Jiazuan Ni , Lingling Chen , Xiaogai Yang , Qiong Liu
Affiliation
|
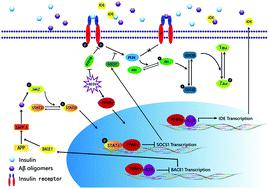
Vanadium compounds have been reported to mimic the anti-diabetes effects of insulin on rodent models, but their effects on Alzheimer's disease (AD) have rarely been explored. In this paper, 9-month-old triple transgenic AD model mice (3×Tg-AD) received bis(ethylmaltolato)oxidovanadium(iv) (BEOV) at doses of 0.2 mmol L-1 (68.4 μg mL-1) and 1.0 mmol L-1 (342 μg mL-1) for 3 months. BEOV at both doses was found to improve contextual memory and spatial learning in AD mice. It also improved glucose metabolism and protected neuronal synapses in the AD brain, as evidenced respectively by 18F-labeled fluoro-deoxyglucose positron emission tomography (18F-FDG-PET) scanning and by transmission electron microscopy. Inhibitory effects of BEOV on β-amyloid (Aβ) plaques and neuronal impairment in the cortex and hippocampus of fluorescent AD mice were visualized three-dimensionally by applying optical clearing technology to brain slices before confocal laser scanning microscopy. Western blot analysis semi-quantitatively revealed the altered levels of Aβ42 in the brains of wildtype, AD, and AD treated with 0.2 and 1.0 mmol L-1 BEOV mice (70.3%, 100%, 83.2% and 56.8% in the hippocampus; 82.4%, 100%, 66.9% and 42% in the cortex, respectively). The mechanism study showed that BEOV increased the expression of peroxisome proliferator-activated receptor γ (PPARγ) (140%, 100%, 142% and 160% in the hippocampus; 167%, 100%, 124% and 133% in the cortex) to inactivate the JAK2/STAT3/SOCS-1 pathway and to block the amyloidogenesis cascade, thus attenuating Aβ-induced insulin resistance in AD models. BEOV also reduced protein tyrosine phosphatase 1B (PTP1B) expression (74.8%, 100%, 76.5% and 53.8% in the hippocampus; 71.8%, 100%, 94.2% and 81.8% in cortex) to promote insulin sensitivity and to stimulate the PI3K/Akt/GSK3β pathway, subsequently reducing tau hyperphosphorylation (phosphorylated tau396 levels were 51.1%, 100%, 56.1% and 50.2% in the hippocampus; 22.2%, 100%, 36.1%, and 24% in the cortex). Our results suggested that BEOV reduced the pathological hallmarks of AD by targeting the pathways of PPARγ and PTP1B in 3×Tg AD mice.
中文翻译:

在三重转基因模型小鼠中,双(乙基麦芽糖基)氧化钒(iv)抑制了阿尔茨海默氏病的发病机理。
据报道,钒化合物可模仿胰岛素对啮齿动物模型的抗糖尿病作用,但很少研究其对阿尔茨海默氏病(AD)的作用。在本文中,9个月大的三重转基因AD模型小鼠(3×Tg-AD)接受双(乙基麦芽甲基)氧化钒(iv)(BEOV)的剂量为0.2 mmol L-1(68.4μgmL-1)和1.0 mmol L-1(342μgmL-1)3个月。发现两种剂量的BEOV均可改善AD小鼠的情境记忆和空间学习。通过18 F标记的氟脱氧葡萄糖正电子发射断层显像(18F-FDG-PET)扫描和透射电子显微镜分别证明,它还改善了AD脑中的葡萄糖代谢并保护了神经元突触。通过在共聚焦激光扫描显微镜下对脑片应用光学清除技术,三维地观察了BEOV对荧光AD小鼠皮质和海马中β淀粉样蛋白(Aβ)斑块和神经元损伤的抑制作用。Western印迹分析半定量显示野生型,AD和用0.2和1.0 mmol L-1 BEOV小鼠治疗的AD大脑中Aβ42的水平发生了改变(海马分别为70.3%,100%,83.2%和56.8%; 82.4分别为%,100%,66.9%和42%)。机制研究表明,BEOV可增加过氧化物酶体增殖物激活受体γ(PPARγ)的表达(海马中140%,100%,142%和160%;皮质中167%,100%,124%和133%)使JAK2 / STAT3 / SOCS-1途径失活并阻止淀粉样蛋白生成级联,因此减弱了AD模型中Aβ诱导的胰岛素抵抗。BEOV还降低了蛋白酪氨酸磷酸酶1B(PTP1B)的表达(海马中74.8%,100%,76.5%和53.8%;皮质中71.8%,100%,94.2%和81.8%),以提高胰岛素敏感性并刺激PI3K。 / Akt /GSK3β途径,随后降低tau过度磷酸化(海马中磷酸化的tau396水平为51.1%,100%,56.1%和50.2%;皮质中为22.2%,100%,36.1%和24%)。我们的结果表明,BEOV通过靶向3xTg AD小鼠中的PPARγ和PTP1B途径来降低AD的病理特征。皮层中的8%)促进胰岛素敏感性并刺激PI3K / Akt /GSK3β途径,随后降低tau过度磷酸化(海马中磷酸化的tau396的水平分别为51.1%,100%,56.1%和50.2%; 22.2%,100%, 36.1%,在皮质中为24%)。我们的结果表明,BEOV通过靶向3xTg AD小鼠中的PPARγ和PTP1B途径来降低AD的病理特征。皮层中的8%)促进胰岛素敏感性并刺激PI3K / Akt /GSK3β途径,随后降低tau过度磷酸化(海马中磷酸化的tau396的水平分别为51.1%,100%,56.1%和50.2%; 22.2%,100%, 36.1%,在皮质中为24%)。我们的结果表明,BEOV通过靶向3xTg AD小鼠中的PPARγ和PTP1B途径来降低AD的病理特征。
更新日期:2020-01-03
中文翻译:

在三重转基因模型小鼠中,双(乙基麦芽糖基)氧化钒(iv)抑制了阿尔茨海默氏病的发病机理。
据报道,钒化合物可模仿胰岛素对啮齿动物模型的抗糖尿病作用,但很少研究其对阿尔茨海默氏病(AD)的作用。在本文中,9个月大的三重转基因AD模型小鼠(3×Tg-AD)接受双(乙基麦芽甲基)氧化钒(iv)(BEOV)的剂量为0.2 mmol L-1(68.4μgmL-1)和1.0 mmol L-1(342μgmL-1)3个月。发现两种剂量的BEOV均可改善AD小鼠的情境记忆和空间学习。通过18 F标记的氟脱氧葡萄糖正电子发射断层显像(18F-FDG-PET)扫描和透射电子显微镜分别证明,它还改善了AD脑中的葡萄糖代谢并保护了神经元突触。通过在共聚焦激光扫描显微镜下对脑片应用光学清除技术,三维地观察了BEOV对荧光AD小鼠皮质和海马中β淀粉样蛋白(Aβ)斑块和神经元损伤的抑制作用。Western印迹分析半定量显示野生型,AD和用0.2和1.0 mmol L-1 BEOV小鼠治疗的AD大脑中Aβ42的水平发生了改变(海马分别为70.3%,100%,83.2%和56.8%; 82.4分别为%,100%,66.9%和42%)。机制研究表明,BEOV可增加过氧化物酶体增殖物激活受体γ(PPARγ)的表达(海马中140%,100%,142%和160%;皮质中167%,100%,124%和133%)使JAK2 / STAT3 / SOCS-1途径失活并阻止淀粉样蛋白生成级联,因此减弱了AD模型中Aβ诱导的胰岛素抵抗。BEOV还降低了蛋白酪氨酸磷酸酶1B(PTP1B)的表达(海马中74.8%,100%,76.5%和53.8%;皮质中71.8%,100%,94.2%和81.8%),以提高胰岛素敏感性并刺激PI3K。 / Akt /GSK3β途径,随后降低tau过度磷酸化(海马中磷酸化的tau396水平为51.1%,100%,56.1%和50.2%;皮质中为22.2%,100%,36.1%和24%)。我们的结果表明,BEOV通过靶向3xTg AD小鼠中的PPARγ和PTP1B途径来降低AD的病理特征。皮层中的8%)促进胰岛素敏感性并刺激PI3K / Akt /GSK3β途径,随后降低tau过度磷酸化(海马中磷酸化的tau396的水平分别为51.1%,100%,56.1%和50.2%; 22.2%,100%, 36.1%,在皮质中为24%)。我们的结果表明,BEOV通过靶向3xTg AD小鼠中的PPARγ和PTP1B途径来降低AD的病理特征。皮层中的8%)促进胰岛素敏感性并刺激PI3K / Akt /GSK3β途径,随后降低tau过度磷酸化(海马中磷酸化的tau396的水平分别为51.1%,100%,56.1%和50.2%; 22.2%,100%, 36.1%,在皮质中为24%)。我们的结果表明,BEOV通过靶向3xTg AD小鼠中的PPARγ和PTP1B途径来降低AD的病理特征。


























 京公网安备 11010802027423号
京公网安备 11010802027423号