当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tuning Allostery through Integration of Disorder to Order with a Residue Network.
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-03 , DOI: 10.1021/acs.biochem.9b01006 Jingheng Wang 1 , Riya Samanta 2 , Gregory Custer 3 , Christopher Look 3 , Silvina Matysiak 3 , Dorothy Beckett 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-03 , DOI: 10.1021/acs.biochem.9b01006 Jingheng Wang 1 , Riya Samanta 2 , Gregory Custer 3 , Christopher Look 3 , Silvina Matysiak 3 , Dorothy Beckett 1
Affiliation
|
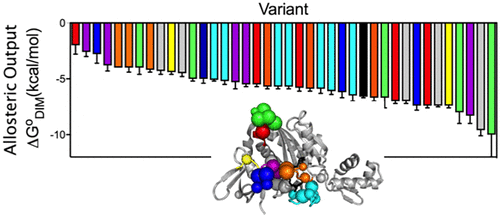
In allostery, a signal from one site in a protein is transmitted to a second site to alter its function. Due to its ubiquity in biology and the potential for its exploitation in drug and protein design, the molecular basis of allosteric communication continues to be the subject of intense research. Although allosterically coupled sites are frequently characterized by disorder, how communication between disordered segments occurs remains obscure. Allosteric activation of Escherichia coli BirA dimerization occurs via coupled distant disorder-to-order transitions. In this work, combined structural and computational studies reveal an extensive residue network in BirA. Substitution of several network residues yields large perturbations to allostery. Force distribution analysis reveals that disruptions to the disorder-to-order transitions through amino acid substitution are manifested in shifts in the energy experienced by network residues as well as alterations in packing of an α-helix that plays a critical role in allostery. The combined results reveal a highly distributed allosteric mechanism that is robust to sequence change.
中文翻译:

通过残基网络与无序整合来优化变构。
在变构中,来自蛋白质中一个位点的信号被传递到另一个位点以改变其功能。由于其在生物学中的普遍性及其在药物和蛋白质设计中的开发潜力,因此变构通讯的分子基础仍然是广泛研究的主题。尽管变构偶联位点通常以疾病为特征,但无序区段之间的通讯方式仍然不清楚。大肠埃希氏菌BirA二聚体的变构活化通过耦合的远距离无序转移发生。在这项工作中,结合结构和计算研究揭示了BirA中广泛的残基网络。替换几个网络残基会产生很大的构象扰动。力分布分析表明,通过氨基酸取代对无序转移的破坏表现为网络残基经历的能量转移以及在变构中起关键作用的α螺旋的堆积变化。结合的结果揭示了高度分布的变构机制,该变构机制对序列改变具有鲁棒性。
更新日期:2020-02-04
中文翻译:

通过残基网络与无序整合来优化变构。
在变构中,来自蛋白质中一个位点的信号被传递到另一个位点以改变其功能。由于其在生物学中的普遍性及其在药物和蛋白质设计中的开发潜力,因此变构通讯的分子基础仍然是广泛研究的主题。尽管变构偶联位点通常以疾病为特征,但无序区段之间的通讯方式仍然不清楚。大肠埃希氏菌BirA二聚体的变构活化通过耦合的远距离无序转移发生。在这项工作中,结合结构和计算研究揭示了BirA中广泛的残基网络。替换几个网络残基会产生很大的构象扰动。力分布分析表明,通过氨基酸取代对无序转移的破坏表现为网络残基经历的能量转移以及在变构中起关键作用的α螺旋的堆积变化。结合的结果揭示了高度分布的变构机制,该变构机制对序列改变具有鲁棒性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号