当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Side Chain Hydrogen-Bonding Interactions within Amyloid-like Fibrils Formed by the Low-Complexity Domain of FUS: Evidence from Solid State Nuclear Magnetic Resonance Spectroscopy.
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.biochem.9b00892 Dylan T Murray 1 , Robert Tycko 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.biochem.9b00892 Dylan T Murray 1 , Robert Tycko 2
Affiliation
|
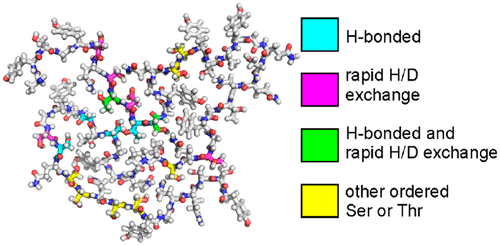
In aqueous solutions, the 214-residue low-complexity domain of the FUS protein (FUS-LC) is known to undergo liquid-liquid phase separation and also to self-assemble into amyloid-like fibrils. In previous work based on solid state nuclear magnetic resonance (ssNMR) methods, a structural model for the FUS-LC fibril core was developed, showing that residues 39-95 form the fibril core. Unlike fibrils formed by amyloid-β peptides, α-synuclein, and other amyloid-forming proteins, the FUS-LC core is largely devoid of purely hydrophobic amino acid side chains. Instead, the core-forming segment contains numerous hydroxyl-bearing residues, including 18 serines, six threonines, and eight tyrosines, suggesting that the FUS-LC fibril structure may be stabilized in part by inter-residue hydrogen bonds among side chain hydroxyl groups. Here we describe ssNMR measurements, performed on 2H,15N,13C-labeled FUS-LC fibrils, that provide new information about the interactions of hydroxyl-bearing residues with one another and with water. The ssNMR data support the involvement of specific serine, threonine, and tyrosine residues in hydrogen-bonding interactions. The data also reveal differences in hydrogen exchange rates with water for different side chain hydroxyl groups, providing information about solvent exposure and penetration of water into the FUS-LC fibril core.
中文翻译:

FUS的低复杂度域形成的淀粉样样原纤维内的侧链氢键相互作用:固态核磁共振波谱的证据。
在水溶液中,已知FUS蛋白(FUS-LC)的214个残基的低复杂性结构域会进行液-液相分离,并且会自组装成淀粉样蛋白原纤维。在先前基于固态核磁共振(ssNMR)方法的工作中,开发了FUS-LC原纤维芯的结构模型,显示残基39-95形成原纤维芯。与由淀粉样蛋白β肽,α-突触核蛋白和其他淀粉样蛋白形成的原纤维不同,FUS-LC核心在很大程度上没有纯疏水性氨基酸侧链。取而代之的是,核心形成链段包含许多带有羟基的残基,包括18个丝氨酸,6个苏氨酸和8个酪氨酸,这表明FUS-LC的原纤维结构可能部分被侧链羟基之间的残基氢键所稳定。在这里,我们描述了在2H,15N,13C标记的FUS-LC原纤维上进行的ssNMR测量,这些测量提供了有关含羟基残基相互之间以及与水之间相互作用的新信息。ssNMR数据支持特定的丝氨酸,苏氨酸和酪氨酸残基参与氢键相互作用。数据还揭示了不同侧链羟基与水的氢交换速率的差异,提供了有关溶剂暴露以及水渗透到FUS-LC原纤维芯中的信息。
更新日期:2020-01-10
中文翻译:

FUS的低复杂度域形成的淀粉样样原纤维内的侧链氢键相互作用:固态核磁共振波谱的证据。
在水溶液中,已知FUS蛋白(FUS-LC)的214个残基的低复杂性结构域会进行液-液相分离,并且会自组装成淀粉样蛋白原纤维。在先前基于固态核磁共振(ssNMR)方法的工作中,开发了FUS-LC原纤维芯的结构模型,显示残基39-95形成原纤维芯。与由淀粉样蛋白β肽,α-突触核蛋白和其他淀粉样蛋白形成的原纤维不同,FUS-LC核心在很大程度上没有纯疏水性氨基酸侧链。取而代之的是,核心形成链段包含许多带有羟基的残基,包括18个丝氨酸,6个苏氨酸和8个酪氨酸,这表明FUS-LC的原纤维结构可能部分被侧链羟基之间的残基氢键所稳定。在这里,我们描述了在2H,15N,13C标记的FUS-LC原纤维上进行的ssNMR测量,这些测量提供了有关含羟基残基相互之间以及与水之间相互作用的新信息。ssNMR数据支持特定的丝氨酸,苏氨酸和酪氨酸残基参与氢键相互作用。数据还揭示了不同侧链羟基与水的氢交换速率的差异,提供了有关溶剂暴露以及水渗透到FUS-LC原纤维芯中的信息。



























 京公网安备 11010802027423号
京公网安备 11010802027423号