当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Axially Chiral Biaryl Monophosphine Oxides Enabled by Palladium/WJ-Phos-Catalyzed Asymmetric Suzuki–Miyaura Cross-coupling
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-01-07 , DOI: 10.1021/acscatal.9b04354 Wangqin Ji 1 , Hai-Hong Wu 1 , Junliang Zhang 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-01-07 , DOI: 10.1021/acscatal.9b04354 Wangqin Ji 1 , Hai-Hong Wu 1 , Junliang Zhang 1, 2
Affiliation
|
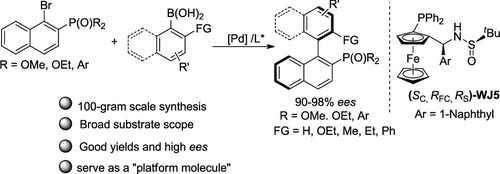
A highly enantioselective palladium/WJ-Phos-catalyzed Suzuki–Miyaura coupling reaction for efficient construction of axially chiral biaryl monophosphine oxides was developed. A series of axially chiral biaryl monophosphine oxides were obtained in good yields and with high enantioselectivities. The practicability of this reaction was validated in the straightforward synthesis of axially chiral biaryl monophosphine ligand and demonstrated by a 100-g-scale synthesis. Moreover, various functionalizations of the product make it as a platform molecule for synthesis of other chiral biaryl phosphines.
中文翻译:

钯/ WJ-磷催化的不对称Suzuki-Miyaura交叉偶联实现的轴向手性联芳基单膦氧化物
开发了一种高对映选择性的钯/ WJ-Phos催化的Suzuki-Miyaura偶联反应,可有效构建轴向手性联芳基单膦氧化物。以高收率和高对映选择性获得了一系列轴向手性联芳基单膦氧化物。该反应的实用性在轴向手性联芳基单膦配体的直接合成中得到验证,并通过100 g规模的合成得到证明。此外,产物的各种官能化使其成为合成其他手性联芳基膦的平台分子。
更新日期:2020-01-07
中文翻译:

钯/ WJ-磷催化的不对称Suzuki-Miyaura交叉偶联实现的轴向手性联芳基单膦氧化物
开发了一种高对映选择性的钯/ WJ-Phos催化的Suzuki-Miyaura偶联反应,可有效构建轴向手性联芳基单膦氧化物。以高收率和高对映选择性获得了一系列轴向手性联芳基单膦氧化物。该反应的实用性在轴向手性联芳基单膦配体的直接合成中得到验证,并通过100 g规模的合成得到证明。此外,产物的各种官能化使其成为合成其他手性联芳基膦的平台分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号