当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Correctly Measuring and Predicting Solubilities of Solvates, Hydrates, and Polymorphs
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-01-27 , DOI: 10.1021/acs.cgd.9b01145 Heiner Veith 1 , Christian Luebbert 1 , Gabriele Sadowski 1
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-01-27 , DOI: 10.1021/acs.cgd.9b01145 Heiner Veith 1 , Christian Luebbert 1 , Gabriele Sadowski 1
Affiliation
|
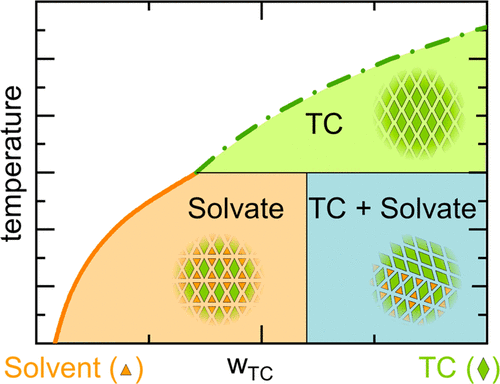
The formation of solvates, hydrates, or different polymorphs significantly alters the physicochemical properties of a target component (TC) (e.g., active pharmaceutical or agrochemical active ingredient), such as solubility and dissolution behavior. Thus, it is very important to know under which conditions a certain solvate, hydrate, or polymorph is formed. This information can be obtained from phase diagrams, which are usually based on solubility measurements. Possible pitfalls in measuring the solubility of TC hydrates or solvates in pure solvents and solvent mixtures are discussed, and strategies for obtaining reliable solubility data are proposed. To substantiate the proposed solubility-measurement strategies, a thermodynamic approach based on the Perturbed-Chain Statistical Associating Fluid Theory for modeling solvate/hydrate solubilities was developed. This approach allows identifying stability regions of solvates/hydrates and predicting solubilities of solvates/hydrates or of polymorphs in pure solvents and solvent mixtures. It was successfully verified for modeling the solubility of the indomethacin-methanol solvate in methanol and of the carbamazepine dihydrate in water. Furthermore, the solubility of carbamazepine dihydrate and theophylline monohydrate in water/ethanol mixtures was predicted in excellent agreement with experimental data.
中文翻译:

正确测量和预测溶剂化物,水合物和多晶型物的溶解度
溶剂化物,水合物或不同多晶型物的形成显着改变了目标组分(TC)(例如活性药物或农用化学活性成分)的物理化学性质,例如溶解度和溶解行为。因此,了解在何种条件下形成某种溶剂化物,水合物或多晶型物非常重要。该信息可以从相图获得,该相图通常基于溶解度测量。讨论了测量TC水合物或溶剂化物在纯溶剂和溶剂混合物中的溶解度时可能遇到的陷阱,并提出了获得可靠溶解度数据的策略。为了证实提议的溶解度测量策略,开发了一种基于扰动链统计缔合流体理论的热力学方法来模拟溶剂化物/水合物的溶解度。这种方法可以确定溶剂化物/水合物的稳定性区域,并预测溶剂化物/水合物或多晶型物在纯溶剂和溶剂混合物中的溶解度。已成功验证了吲哚美辛-甲醇溶剂化物在甲醇中和卡马西平二水合物在水中的溶解度模型。此外,预测了卡马西平二水合物和茶碱一水合物在水/乙醇混合物中的溶解度,与实验数据非常吻合。已成功验证了吲哚美辛-甲醇溶剂化物在甲醇中和卡马西平二水合物在水中的溶解度模型。此外,预测了卡马西平二水合物和茶碱一水合物在水/乙醇混合物中的溶解度,与实验数据非常吻合。已成功验证了吲哚美辛-甲醇溶剂化物在甲醇中和卡马西平二水合物在水中的溶解度模型。此外,预测了卡马西平二水合物和茶碱一水合物在水/乙醇混合物中的溶解度,与实验数据非常吻合。
更新日期:2020-01-27
中文翻译:

正确测量和预测溶剂化物,水合物和多晶型物的溶解度
溶剂化物,水合物或不同多晶型物的形成显着改变了目标组分(TC)(例如活性药物或农用化学活性成分)的物理化学性质,例如溶解度和溶解行为。因此,了解在何种条件下形成某种溶剂化物,水合物或多晶型物非常重要。该信息可以从相图获得,该相图通常基于溶解度测量。讨论了测量TC水合物或溶剂化物在纯溶剂和溶剂混合物中的溶解度时可能遇到的陷阱,并提出了获得可靠溶解度数据的策略。为了证实提议的溶解度测量策略,开发了一种基于扰动链统计缔合流体理论的热力学方法来模拟溶剂化物/水合物的溶解度。这种方法可以确定溶剂化物/水合物的稳定性区域,并预测溶剂化物/水合物或多晶型物在纯溶剂和溶剂混合物中的溶解度。已成功验证了吲哚美辛-甲醇溶剂化物在甲醇中和卡马西平二水合物在水中的溶解度模型。此外,预测了卡马西平二水合物和茶碱一水合物在水/乙醇混合物中的溶解度,与实验数据非常吻合。已成功验证了吲哚美辛-甲醇溶剂化物在甲醇中和卡马西平二水合物在水中的溶解度模型。此外,预测了卡马西平二水合物和茶碱一水合物在水/乙醇混合物中的溶解度,与实验数据非常吻合。已成功验证了吲哚美辛-甲醇溶剂化物在甲醇中和卡马西平二水合物在水中的溶解度模型。此外,预测了卡马西平二水合物和茶碱一水合物在水/乙醇混合物中的溶解度,与实验数据非常吻合。

























 京公网安备 11010802027423号
京公网安备 11010802027423号