当前位置:
X-MOL 学术
›
Gene Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Integrating gene delivery and gene-editing technologies by adenoviral vector transfer of optimized CRISPR-Cas9 components.
Gene Therapy ( IF 5.1 ) Pub Date : 2020-01-03 , DOI: 10.1038/s41434-019-0119-y Ignazio Maggio 1, 2 , Hidde A Zittersteijn 2 , Qian Wang 2 , Jin Liu 2 , Josephine M Janssen 2 , Ivan Toral Ojeda 2 , Silvère M van der Maarel 3 , Arjan C Lankester 1 , Rob C Hoeben 2 , Manuel A F V Gonçalves 2
Gene Therapy ( IF 5.1 ) Pub Date : 2020-01-03 , DOI: 10.1038/s41434-019-0119-y Ignazio Maggio 1, 2 , Hidde A Zittersteijn 2 , Qian Wang 2 , Jin Liu 2 , Josephine M Janssen 2 , Ivan Toral Ojeda 2 , Silvère M van der Maarel 3 , Arjan C Lankester 1 , Rob C Hoeben 2 , Manuel A F V Gonçalves 2
Affiliation
|
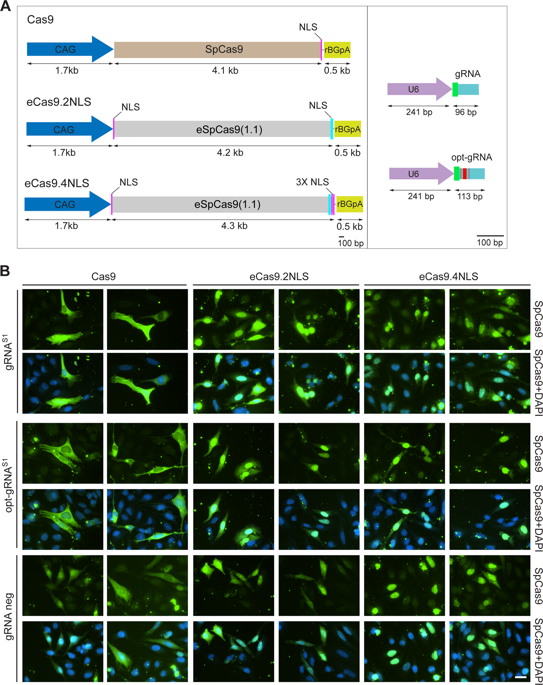
Enhancing the intracellular delivery and performance of RNA-guided CRISPR-Cas9 nucleases (RGNs) remains in demand. Here, we show that nuclear translocation of commonly used Streptococcus pyogenes Cas9 (SpCas9) proteins is suboptimal. Hence, we generated eCas9.4NLS by endowing the high-specificity eSpCas9(1.1) nuclease (eCas9.2NLS) with additional nuclear localization signals (NLSs). We demonstrate that eCas9.4NLS coupled to prototypic or optimized guide RNAs achieves efficient targeted DNA cleavage and probe the performance of SpCas9 proteins with different NLS compositions at target sequences embedded in heterochromatin versus euchromatin. Moreover, after adenoviral vector (AdV)-mediated transfer of SpCas9 expression units, unbiased quantitative immunofluorescence microscopy revealed 2.3-fold higher eCas9.4NLS nuclear enrichment levels than those observed for high-specificity eCas9.2NLS. This improved nuclear translocation yielded in turn robust gene editing after nonhomologous end joining repair of targeted double-stranded DNA breaks. In particular, AdV delivery of eCas9.4NLS into muscle progenitor cells resulted in significantly higher editing frequencies at defective DMD alleles causing Duchenne muscular dystrophy (DMD) than those achieved by AdVs encoding the parental, eCas9.2NLS, protein. In conclusion, this work provides a strong rationale for integrating viral vector and optimized gene-editing technologies to bring about enhanced RGN delivery and performance.
中文翻译:

通过优化 CRISPR-Cas9 组件的腺病毒载体转移整合基因递送和基因编辑技术。
仍然需要增强 RNA 引导的 CRISPR-Cas9 核酸酶 (RGN) 的细胞内递送和性能。在这里,我们表明常用的化脓性链球菌 Cas9 (SpCas9) 蛋白的核易位是次优的。因此,我们通过赋予高特异性 eSpCas9(1.1) 核酸酶 (eCas9.2NLS) 额外的核定位信号 (NLS) 来生成 eCas9.4NLS。我们证明 eCas9.4NLS 与原型或优化的引导 RNA 耦合实现了高效的靶向 DNA 切割,并在嵌入异染色质与常染色质的靶序列中探测具有不同 NLS 组成的 SpCas9 蛋白的性能。此外,在腺病毒载体 (AdV) 介导的 SpCas9 表达单位转移后,无偏定量免疫荧光显微镜显示 eCas9 高 2.3 倍。4NLS 核富集水平比高特异性 eCas9.2NLS 观察到的水平高。在靶向双链 DNA 断裂的非同源末端连接修复后,这种改进的核易位反过来产生了强大的基因编辑。特别是,与通过编码亲本 eCas9.2NLS 蛋白质的 AdV 实现的编辑频率相比,eCas9.4NLS 向肌肉祖细胞的 AdV 递送导致导致杜氏肌营养不良症 (DMD) 的缺陷 DMD 等位基因的编辑频率显着更高。总之,这项工作为整合病毒载体和优化的基因编辑技术以实现增强的 RGN 交付和性能提供了强有力的理由。在靶向双链 DNA 断裂的非同源末端连接修复后,这种改进的核易位反过来产生了强大的基因编辑。特别是,与通过编码亲本 eCas9.2NLS 蛋白质的 AdV 实现的编辑频率相比,eCas9.4NLS 向肌肉祖细胞的 AdV 递送导致导致杜氏肌营养不良症 (DMD) 的缺陷 DMD 等位基因的编辑频率显着更高。总之,这项工作为整合病毒载体和优化的基因编辑技术以实现增强的 RGN 交付和性能提供了强有力的理由。在靶向双链 DNA 断裂的非同源末端连接修复后,这种改进的核易位反过来产生了强大的基因编辑。特别是,与通过编码亲本 eCas9.2NLS 蛋白质的 AdV 实现的编辑频率相比,eCas9.4NLS 向肌肉祖细胞的 AdV 递送导致导致杜氏肌营养不良症 (DMD) 的缺陷 DMD 等位基因的编辑频率显着更高。总之,这项工作为整合病毒载体和优化的基因编辑技术以实现增强的 RGN 交付和性能提供了强有力的理由。
更新日期:2020-01-03
中文翻译:

通过优化 CRISPR-Cas9 组件的腺病毒载体转移整合基因递送和基因编辑技术。
仍然需要增强 RNA 引导的 CRISPR-Cas9 核酸酶 (RGN) 的细胞内递送和性能。在这里,我们表明常用的化脓性链球菌 Cas9 (SpCas9) 蛋白的核易位是次优的。因此,我们通过赋予高特异性 eSpCas9(1.1) 核酸酶 (eCas9.2NLS) 额外的核定位信号 (NLS) 来生成 eCas9.4NLS。我们证明 eCas9.4NLS 与原型或优化的引导 RNA 耦合实现了高效的靶向 DNA 切割,并在嵌入异染色质与常染色质的靶序列中探测具有不同 NLS 组成的 SpCas9 蛋白的性能。此外,在腺病毒载体 (AdV) 介导的 SpCas9 表达单位转移后,无偏定量免疫荧光显微镜显示 eCas9 高 2.3 倍。4NLS 核富集水平比高特异性 eCas9.2NLS 观察到的水平高。在靶向双链 DNA 断裂的非同源末端连接修复后,这种改进的核易位反过来产生了强大的基因编辑。特别是,与通过编码亲本 eCas9.2NLS 蛋白质的 AdV 实现的编辑频率相比,eCas9.4NLS 向肌肉祖细胞的 AdV 递送导致导致杜氏肌营养不良症 (DMD) 的缺陷 DMD 等位基因的编辑频率显着更高。总之,这项工作为整合病毒载体和优化的基因编辑技术以实现增强的 RGN 交付和性能提供了强有力的理由。在靶向双链 DNA 断裂的非同源末端连接修复后,这种改进的核易位反过来产生了强大的基因编辑。特别是,与通过编码亲本 eCas9.2NLS 蛋白质的 AdV 实现的编辑频率相比,eCas9.4NLS 向肌肉祖细胞的 AdV 递送导致导致杜氏肌营养不良症 (DMD) 的缺陷 DMD 等位基因的编辑频率显着更高。总之,这项工作为整合病毒载体和优化的基因编辑技术以实现增强的 RGN 交付和性能提供了强有力的理由。在靶向双链 DNA 断裂的非同源末端连接修复后,这种改进的核易位反过来产生了强大的基因编辑。特别是,与通过编码亲本 eCas9.2NLS 蛋白质的 AdV 实现的编辑频率相比,eCas9.4NLS 向肌肉祖细胞的 AdV 递送导致导致杜氏肌营养不良症 (DMD) 的缺陷 DMD 等位基因的编辑频率显着更高。总之,这项工作为整合病毒载体和优化的基因编辑技术以实现增强的 RGN 交付和性能提供了强有力的理由。



























 京公网安备 11010802027423号
京公网安备 11010802027423号