当前位置:
X-MOL 学术
›
Gene Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heat-shrinking DNA nanoparticles for in vivo gene delivery.
Gene Therapy ( IF 5.1 ) Pub Date : 2020-01-03 , DOI: 10.1038/s41434-019-0117-0 Basil Mathew 1 , Raghu Ramanathan 1 , Nathan A Delvaux 1 , Jacob Poliskey 1 , Kevin G Rice 1
Gene Therapy ( IF 5.1 ) Pub Date : 2020-01-03 , DOI: 10.1038/s41434-019-0117-0 Basil Mathew 1 , Raghu Ramanathan 1 , Nathan A Delvaux 1 , Jacob Poliskey 1 , Kevin G Rice 1
Affiliation
|
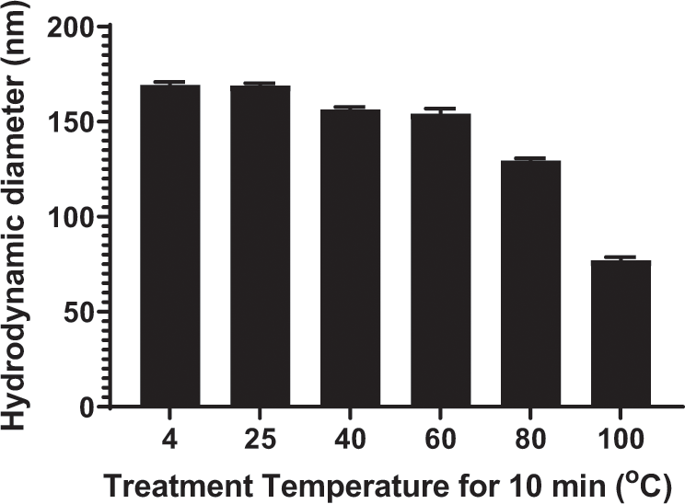
The particle size of a PEG-peptide DNA nanoparticle is a key determinant of biodistribution following i.v. dosing. DNA nanoparticles of <100 nm in diameter are sufficiently small to cross through fenestrated endothelial cells to target hepatocytes in the liver. In addition, DNA nanoparticles must be close to charge-neutral to avoid recognition and binding to scavenger receptors found on Kupffer cells and endothelial cells in the liver. In the present study, we demonstrate an approach to heat shrink DNA nanoparticles to reduce their size to <100 nm to target hepatocytes. An optimized protocol heated plasmid DNA at 100 °C for 10 min resulting in partial denaturation. The immediate addition of a polyacridine PEG-peptide followed by cooling to room temperature resulted in heat-shrunken DNA nanoparticles that were ~70 nm in diameter compared with 170 nm when heating was omitted. Heat shrinking resulted in the conversion of supercoiled DNA into open circular to remove strain during compaction. Heat-shrunken DNA nanoparticles were stable to freeze-drying and reconstitution in saline. Hydrodynamic dosing established that 70 nm heat-shrunken DNA nanoparticles efficiently expressed luciferase in mouse liver. Biodistribution studies revealed that 70 nm DNA nanoparticles are rapidly and transiently taken up by liver whereas 170 nm DNA nanoparticles avoid liver uptake due to their larger size. The results provide a new approach to decrease the size of polyacridine PEG-peptide DNA nanoparticles to allow penetration of the fenestrated endothelium of the liver for the purpose of transfecting hepatocytes in vivo.
中文翻译:

用于体内基因传递的热收缩 DNA 纳米粒子。
PEG-肽DNA纳米颗粒的粒径是静脉给药后生物分布的关键决定因素。直径 <100 nm 的 DNA 纳米颗粒足够小,可以穿过有孔的内皮细胞,以靶向肝脏中的肝细胞。此外,DNA 纳米粒子必须接近电荷中性,以避免识别和结合在肝脏中的库普弗细胞和内皮细胞上发现的清道夫受体。在本研究中,我们展示了一种热收缩 DNA 纳米颗粒的方法,以将其尺寸减小到 <100 nm,以靶向肝细胞。优化的方案将质粒 DNA 在 100°C 加热 10 分钟,导致部分变性。立即添加聚吖啶 PEG 肽,然后冷却至室温,导致热收缩的 DNA 纳米颗粒直径为 ~70 nm,而省略加热时为 170 nm。热收缩导致超螺旋 DNA 转化为开环,以消除压实过程中的应变。热收缩的 DNA 纳米粒子对冷冻干燥和在盐水中重建是稳定的。流体动力学剂量确定 70 nm 热收缩 DNA 纳米粒子在小鼠肝脏中有效表达荧光素酶。生物分布研究表明,70 nm DNA 纳米颗粒会被肝脏快速和瞬时吸收,而 170 nm DNA 纳米颗粒由于尺寸较大而避免肝脏吸收。
更新日期:2020-01-03
中文翻译:

用于体内基因传递的热收缩 DNA 纳米粒子。
PEG-肽DNA纳米颗粒的粒径是静脉给药后生物分布的关键决定因素。直径 <100 nm 的 DNA 纳米颗粒足够小,可以穿过有孔的内皮细胞,以靶向肝脏中的肝细胞。此外,DNA 纳米粒子必须接近电荷中性,以避免识别和结合在肝脏中的库普弗细胞和内皮细胞上发现的清道夫受体。在本研究中,我们展示了一种热收缩 DNA 纳米颗粒的方法,以将其尺寸减小到 <100 nm,以靶向肝细胞。优化的方案将质粒 DNA 在 100°C 加热 10 分钟,导致部分变性。立即添加聚吖啶 PEG 肽,然后冷却至室温,导致热收缩的 DNA 纳米颗粒直径为 ~70 nm,而省略加热时为 170 nm。热收缩导致超螺旋 DNA 转化为开环,以消除压实过程中的应变。热收缩的 DNA 纳米粒子对冷冻干燥和在盐水中重建是稳定的。流体动力学剂量确定 70 nm 热收缩 DNA 纳米粒子在小鼠肝脏中有效表达荧光素酶。生物分布研究表明,70 nm DNA 纳米颗粒会被肝脏快速和瞬时吸收,而 170 nm DNA 纳米颗粒由于尺寸较大而避免肝脏吸收。



























 京公网安备 11010802027423号
京公网安备 11010802027423号