Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Trends in licence approvals for ophthalmic medicines in the United Kingdom
Eye ( IF 3.9 ) Pub Date : 2020-01-03 , DOI: 10.1038/s41433-019-0758-7 Peter J Morgan-Warren 1 , Jiten B Morarji 1
Eye ( IF 3.9 ) Pub Date : 2020-01-03 , DOI: 10.1038/s41433-019-0758-7 Peter J Morgan-Warren 1 , Jiten B Morarji 1
Affiliation
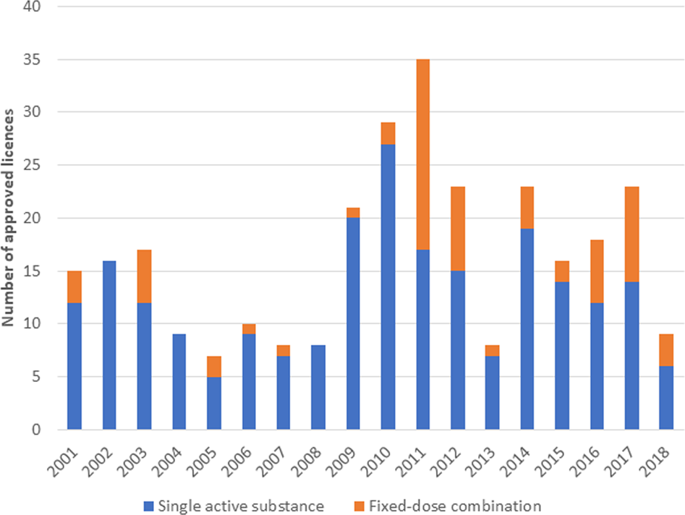
|
Objectives The grant of marketing authorisation (MA) for new medicines requires comprehensive assessment by regulatory authorities. This study sought to identify ophthalmic medicines granted United Kingdom MA and consider trends in licence approvals. Methods This retrospective study reviewed published lists of products granted MA by the UK Medicines & Healthcare products Regulatory Agency between January 2001 and December 2018, inclusive. Eye drops and medicinal products intended for ophthalmic use were identified. All regulatory data sources consulted are in the public domain. Data analyses were descriptive. Results A total of 295 MAs were issued for ophthalmic products between 2001 and 2018, inclusive. Of these 229 (78%) were for single-active substances and 66 (22%) fixed-dose combination (FDC). Approvals for products with single-active substance included ocular hypotensives (115; 50%), antibiotics (48; 22%), allergy medicines (30; 13%), lubricants (18; 8%) and anti-inflammatories (11; 5%). Approvals for FDCs were predominantly ocular hypotensives (56; 85%), with timolol combined with carbonic anhydrase inhibitors (27; 48%) and prostaglandin analogues (26; 46%) accounting for the majority of glaucoma FDCs. Other FDCs were approved for antibiotic/inflammatory (5; 7.5%), pupillary mydriasis (2; 3%), allergy (2; 3%) and ocular surface lubrication (1; 1.5%) products. A median of 16 licences were approved per year (range 7 [2005] to 35 [2011]). Conclusions The majority of MAs granted were for single-agent products, particularly ocular hypotensives and antibiotic preparations. Most products were generic versions of well-established active substances. A trend for increased approvals for FDCs is evident, particularly for the treatment of raised IOP.
中文翻译:

英国眼科药物许可审批趋势
目标 授予新药上市许可 (MA) 需要监管机构进行全面评估。本研究旨在确定获得英国 MA 的眼科药物,并考虑许可批准的趋势。方法 这项回顾性研究回顾了 2001 年 1 月至 2018 年 12 月(含)期间英国药品和保健品监管局授予 MA 的已发布产品清单。确定了用于眼科用途的滴眼液和医药产品。查阅的所有监管数据来源均属于公共领域。数据分析是描述性的。结果 2001 年至 2018 年(包括 2018 年)共为眼科产品签发了 295 份 MA。其中 229 个(78%)用于单一活性物质,66 个(22%)用于固定剂量组合(FDC)。对含有单一活性物质的产品的批准包括降眼压药(115;50%)、抗生素(48;22%)、抗过敏药(30;13%)、润滑剂(18;8%)和消炎药(11;5 %)。批准的 FDC 主要是降眼压药(56;85%),噻吗洛尔联合碳酸酐酶抑制剂(27;48%)和前列腺素类似物(26;46%)占青光眼 FDC 的大部分。其他 FDC 被批准用于抗生素/炎症(5;7.5%)、瞳孔散瞳(2;3%)、过敏(2;3%)和眼表润滑(1;1.5%)产品。每年批准的许可证中位数为 16 个(范围为 7 [2005] 至 35 [2011])。结论 授予的大多数 MA 用于单药产品,特别是降眼压药和抗生素制剂。大多数产品是公认的活性物质的通用版本。FDC 批准增加的趋势很明显,特别是用于治疗眼压升高。
更新日期:2020-01-03
中文翻译:

英国眼科药物许可审批趋势
目标 授予新药上市许可 (MA) 需要监管机构进行全面评估。本研究旨在确定获得英国 MA 的眼科药物,并考虑许可批准的趋势。方法 这项回顾性研究回顾了 2001 年 1 月至 2018 年 12 月(含)期间英国药品和保健品监管局授予 MA 的已发布产品清单。确定了用于眼科用途的滴眼液和医药产品。查阅的所有监管数据来源均属于公共领域。数据分析是描述性的。结果 2001 年至 2018 年(包括 2018 年)共为眼科产品签发了 295 份 MA。其中 229 个(78%)用于单一活性物质,66 个(22%)用于固定剂量组合(FDC)。对含有单一活性物质的产品的批准包括降眼压药(115;50%)、抗生素(48;22%)、抗过敏药(30;13%)、润滑剂(18;8%)和消炎药(11;5 %)。批准的 FDC 主要是降眼压药(56;85%),噻吗洛尔联合碳酸酐酶抑制剂(27;48%)和前列腺素类似物(26;46%)占青光眼 FDC 的大部分。其他 FDC 被批准用于抗生素/炎症(5;7.5%)、瞳孔散瞳(2;3%)、过敏(2;3%)和眼表润滑(1;1.5%)产品。每年批准的许可证中位数为 16 个(范围为 7 [2005] 至 35 [2011])。结论 授予的大多数 MA 用于单药产品,特别是降眼压药和抗生素制剂。大多数产品是公认的活性物质的通用版本。FDC 批准增加的趋势很明显,特别是用于治疗眼压升高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号