Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Immunomodulation with pomalidomide at early lymphocyte recovery after induction chemotherapy in newly diagnosed AML and high-risk MDS.
Leukemia ( IF 11.4 ) Pub Date : 2020-01-03 , DOI: 10.1038/s41375-019-0693-4 Joshua F Zeidner 1 , Hanna A Knaus 2, 3 , Amer M Zeidan 4 , Amanda L Blackford 2 , Raul Montiel-Esparza 2 , Hubert Hackl 5 , Gabrielle T Prince 2 , Lukasz P Gondek 2 , Gabriel Ghiaur 2 , Margaret M Showel 2 , Amy E DeZern 2 , Keith W Pratz 2 , B Douglas Smith 2 , Mark J Levis 2 , Steven Gore 4 , Catherine C Coombs 1 , Matthew C Foster 1 , Howard Streicher 6 , Judith E Karp 2 , Leo Luznik 2 , Ivana Gojo 2
Leukemia ( IF 11.4 ) Pub Date : 2020-01-03 , DOI: 10.1038/s41375-019-0693-4 Joshua F Zeidner 1 , Hanna A Knaus 2, 3 , Amer M Zeidan 4 , Amanda L Blackford 2 , Raul Montiel-Esparza 2 , Hubert Hackl 5 , Gabrielle T Prince 2 , Lukasz P Gondek 2 , Gabriel Ghiaur 2 , Margaret M Showel 2 , Amy E DeZern 2 , Keith W Pratz 2 , B Douglas Smith 2 , Mark J Levis 2 , Steven Gore 4 , Catherine C Coombs 1 , Matthew C Foster 1 , Howard Streicher 6 , Judith E Karp 2 , Leo Luznik 2 , Ivana Gojo 2
Affiliation
|
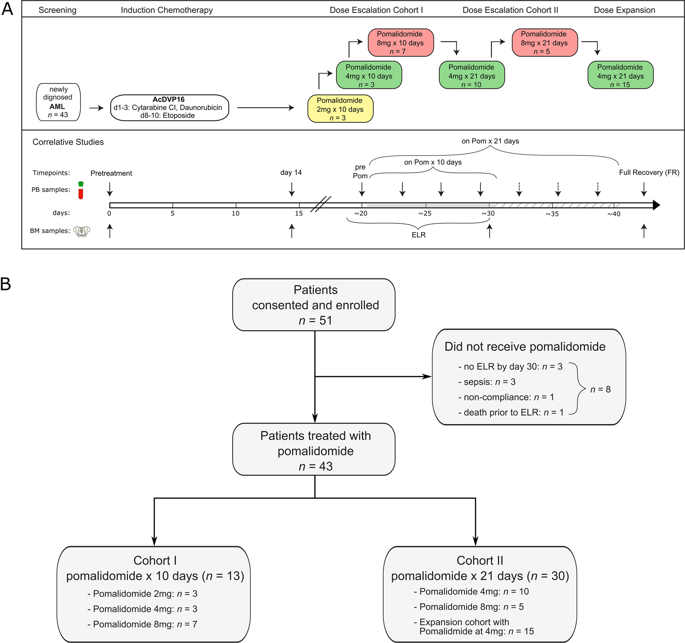
An immunosuppressive microenvironment promoting leukemia cell immune escape plays an important role in the pathogenesis of AML. Through its interaction with cereblon, a substrate receptor for the E3 ubiquitin ligase complex, pomalidomide leads to selective ubiquitination of transcription factors Aiolos and Ikaros thereby promoting immune modulation. In this phase I trial, 51 newly diagnosed non-favorable risk AML and high-risk MDS patients were enrolled and treated with AcDVP16 (cytarabine 667 mg/m2/day IV continuous infusion days 1-3, daunorubicin 45 mg/m2 IV days 1-3, etoposide 400 mg/m2 IV days 8-10) induction therapy followed by dose- and duration-escalation pomalidomide beginning at early lymphocyte recovery. Forty-three patients (AML: n = 39, MDS: n = 4) received pomalidomide. The maximum tolerated dose of pomalidomide was 4 mg for 21 consecutive days. The overall complete remission (CR + CRi) rate, median overall survival, and disease-free survival were 75%, 27.1 and 20.6 months, respectively. Subset analyses revealed 86% CR/CRi rate in AML patients with unfavorable-risk karyotype treated with pomalidomide. Pomalidomide significantly decreased Aiolos expression in both CD4+ and CD8+ peripheral blood and bone marrow T cells, promoted T cell differentiation, proliferation, and heightened their cytokine production. Finally, pomalidomide induced distinct gene expression changes in immune function-related ontologies in CD4+ and CD8+ T cells.
中文翻译:

新诊断的 AML 和高危 MDS 诱导化疗后早期淋巴细胞恢复时使用泊马度胺进行免疫调节。
促进白血病细胞免疫逃逸的免疫抑制微环境在AML的发病机制中发挥着重要作用。通过与 E3 泛素连接酶复合物的底物受体 cereblon 相互作用,泊马度胺导致转录因子 Aiolos 和 Ikaros 选择性泛素化,从而促进免疫调节。在这项 I 期试验中,入组了 51 名新诊断的非有利风险 AML 和高危 MDS 患者,并接受 AcDVP16 治疗(阿糖胞苷 667 mg/m2/天 IV 连续输注第 1-3 天,柔红霉素 45 mg/m2 IV 第 1 天) -3,依托泊苷 400 mg/m2 IV,第 8-10 天)诱导治疗,然后从淋巴细胞恢复早期开始增加泊马度胺的剂量和持续时间。43 名患者(AML:n = 39,MDS:n = 4)接受泊马度胺治疗。泊马度胺的最大耐受剂量为连续 21 天 4 mg。总体完全缓解(CR + CRi)率、中位总体生存率和无病生存率分别为 75%、27.1 和 20.6 个月。子集分析显示,接受泊马度胺治疗的具有不利风险核型的 AML 患者的 CR/CRi 率为 86%。泊马度胺显着降低 Aiolos 在外周血和骨髓 T 细胞中 CD4+ 和 CD8+ 的表达,促进 T 细胞分化、增殖,并提高其细胞因子的产生。最后,泊马度胺诱导 CD4+ 和 CD8+ T 细胞中免疫功能相关本体的明显基因表达变化。子集分析显示,接受泊马度胺治疗的具有不利风险核型的 AML 患者的 CR/CRi 率为 86%。泊马度胺显着降低 Aiolos 在外周血和骨髓 T 细胞中 CD4+ 和 CD8+ 的表达,促进 T 细胞分化、增殖,并提高其细胞因子的产生。最后,泊马度胺诱导 CD4+ 和 CD8+ T 细胞中免疫功能相关本体的明显基因表达变化。子集分析显示,接受泊马度胺治疗的具有不利风险核型的 AML 患者的 CR/CRi 率为 86%。泊马度胺显着降低 Aiolos 在外周血和骨髓 T 细胞中 CD4+ 和 CD8+ 的表达,促进 T 细胞分化、增殖,并提高其细胞因子的产生。最后,泊马度胺诱导 CD4+ 和 CD8+ T 细胞中免疫功能相关本体的明显基因表达变化。
更新日期:2020-01-04
中文翻译:

新诊断的 AML 和高危 MDS 诱导化疗后早期淋巴细胞恢复时使用泊马度胺进行免疫调节。
促进白血病细胞免疫逃逸的免疫抑制微环境在AML的发病机制中发挥着重要作用。通过与 E3 泛素连接酶复合物的底物受体 cereblon 相互作用,泊马度胺导致转录因子 Aiolos 和 Ikaros 选择性泛素化,从而促进免疫调节。在这项 I 期试验中,入组了 51 名新诊断的非有利风险 AML 和高危 MDS 患者,并接受 AcDVP16 治疗(阿糖胞苷 667 mg/m2/天 IV 连续输注第 1-3 天,柔红霉素 45 mg/m2 IV 第 1 天) -3,依托泊苷 400 mg/m2 IV,第 8-10 天)诱导治疗,然后从淋巴细胞恢复早期开始增加泊马度胺的剂量和持续时间。43 名患者(AML:n = 39,MDS:n = 4)接受泊马度胺治疗。泊马度胺的最大耐受剂量为连续 21 天 4 mg。总体完全缓解(CR + CRi)率、中位总体生存率和无病生存率分别为 75%、27.1 和 20.6 个月。子集分析显示,接受泊马度胺治疗的具有不利风险核型的 AML 患者的 CR/CRi 率为 86%。泊马度胺显着降低 Aiolos 在外周血和骨髓 T 细胞中 CD4+ 和 CD8+ 的表达,促进 T 细胞分化、增殖,并提高其细胞因子的产生。最后,泊马度胺诱导 CD4+ 和 CD8+ T 细胞中免疫功能相关本体的明显基因表达变化。子集分析显示,接受泊马度胺治疗的具有不利风险核型的 AML 患者的 CR/CRi 率为 86%。泊马度胺显着降低 Aiolos 在外周血和骨髓 T 细胞中 CD4+ 和 CD8+ 的表达,促进 T 细胞分化、增殖,并提高其细胞因子的产生。最后,泊马度胺诱导 CD4+ 和 CD8+ T 细胞中免疫功能相关本体的明显基因表达变化。子集分析显示,接受泊马度胺治疗的具有不利风险核型的 AML 患者的 CR/CRi 率为 86%。泊马度胺显着降低 Aiolos 在外周血和骨髓 T 细胞中 CD4+ 和 CD8+ 的表达,促进 T 细胞分化、增殖,并提高其细胞因子的产生。最后,泊马度胺诱导 CD4+ 和 CD8+ T 细胞中免疫功能相关本体的明显基因表达变化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号