Protein & Peptide Letters ( IF 1.6 ) Pub Date : 2020-05-31 , DOI: 10.2174/0929866526666191104145511 Pritha Mandal 1 , Anisur R Molla 2
|
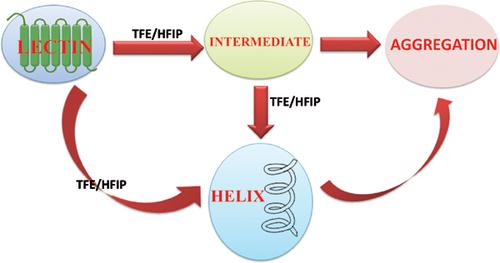
Use of organic molecules as co-solvent with water, the ubiquitous biological solvent, to perturb the structure of proteins is popular in the research area of protein structure and folding. These organic co-solvents are believed to somehow mimic the environment near the cell membrane. Apart from that they induce non-native states which can be present in the protein folding pathway or those states also may be representative of the off pathway structures leading to amyloid formation, responsible for various fatal diseases. In this review, we shall focus on organic co-solvent induced structure perturbation of various members of lectin family. Lectins are excellent model systems for protein folding study because of its wide occurrence, diverse structure and versatile biological functions. Lectins were mainly perturbed by two fluoroalcohols – 2,2,2- trifluoroethanol and 1,1,1,3,3,3-hexafluoroisopropanol whereas glycerol, ethylene glycol and polyethylene glycols were used in some cases. Overall, all native lectins were denatured by alcohols and most of the denatured lectins have predominant helical secondary structure. But characterization of the helical states and the transition pathway for various lectins revealed diverse result.
中文翻译:

蛋白质结构的溶剂扰动-凝集素研究综述。
在蛋白质结构和折叠的研究领域中,使用有机分子与作为普遍存在的生物溶剂的水共溶剂来干扰蛋白质的结构。这些有机助溶剂被认为以某种方式模仿了细胞膜附近的环境。除此之外,它们会诱导可能存在于蛋白质折叠途径中的非天然状态,或者那些状态也可能代表导致淀粉样蛋白形成的非途径结构,从而导致各种致命疾病。在这篇综述中,我们将重点研究凝集素家族各个成员的有机助溶剂诱导的结构扰动。凝集素因其广泛存在,多样的结构和多种生物学功能而成为蛋白质折叠研究的优秀模型系统。凝集素主要受到两种氟代醇的干扰– 2,2,2-三氟乙醇和1,1,1,1,3,3,3-六氟异丙醇,而在某些情况下使用甘油,乙二醇和聚乙二醇。总的来说,所有天然凝集素都被酒精变性,大多数变性的凝集素主要具有螺旋二级结构。但是对各种凝集素的螺旋状态和过渡途径的表征揭示了不同的结果。



























 京公网安备 11010802027423号
京公网安备 11010802027423号