Protein & Peptide Letters ( IF 1.6 ) Pub Date : 2020-07-31 , DOI: 10.2174/0929866526666191125101122 Sauradipta Banerjee 1
|
Background: Non-enzymatic protein glycation is involved in structure and stability changes that impair protein functionality, resulting in several human diseases, such as diabetes and amyloidotic neuropathies (Alzheimer’s disease, Parkinson’s disease and Andrade’s syndrome). Glyoxal, an endogenous reactive oxoaldehyde, increases in diabetes and reacts with several proteins to form advanced glycation end products through Maillard-like reaction.
Objective: Human hemoglobin, the most abundant protein in blood cells is subjected to nonenzymatic modification by reactive oxoaldehydes in diabetic condition. In the present study, the effect of a low concentration of glyoxal (5 μM) on hemoglobin (10 μM) has been investigated following a period of 30 days incubation in vitro.
Methods: Different techniques, mostly biophysical and spectroscopic (e.g. circular dichroism, differential scanning calorimetric study, dynamic light scattering, mass spectrometry, etc.) were used to study glyoxal-induced changes of hemoglobin.
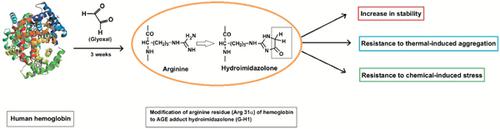
Results: Glyoxal-treated hemoglobin exhibits decreased absorbance around 280 nm, decreased fluorescence and reduced surface hydrophobicity compared to normal hemoglobin. Glyoxal treatment enhances the stability of hemoglobin and lowers its susceptibility to thermal aggregation compared to control hemoglobin as seen by different studies. Finally, peptide mass fingerprinting study showed glyoxal to modify an arginine residue of α-chain of hemoglobin (Arg-31α) to hydroimidazolone.
Conclusion: Increased level of glyoxal in diabetes mellitus as well as its high reactivity may cause modifications of the heme protein. Thus, considering the significance of glyoxal-induced protein modification under physiological conditions, the observation appears clinically relevant in terms of understanding hydroimidazolone-mediated protein modification under in vivo conditions.
中文翻译:

乙二醛修饰对血红蛋白的关键精氨酸残基(Arg-31α)的影响:先进的糖化终产物的生理学意义体外研究。
背景:非酶蛋白糖基化参与结构和稳定性的改变,损害蛋白的功能,导致多种人类疾病,例如糖尿病和淀粉样变性神经病(阿尔茨海默氏病,帕金森氏病和安德拉德综合症)。乙二醛是一种内源性的反应性乙醛,在糖尿病中会增加,并通过美拉德样反应与几种蛋白质反应形成高级糖基化终产物。
目的:在糖尿病条件下,人血红蛋白是血细胞中最丰富的蛋白质,会通过反应性乙醛进行非酶修饰。在本研究中,已经研究了体外孵育30天后低浓度乙二醛(5μM)对血红蛋白(10μM)的影响。
方法:采用不同的技术,主要是生物物理和光谱学(例如,圆二色性,差示扫描量热法,动态光散射,质谱法等)研究乙二醛引起的血红蛋白变化。
结果:与正常的血红蛋白相比,乙二醛处理的血红蛋白在280 nm处的吸光度降低,荧光降低,表面疏水性降低。与对照血红蛋白相比,乙二醛处理可增强血红蛋白的稳定性,并降低其对热聚集的敏感性,如不同研究所见。最后,肽质量指纹图谱研究表明乙二醛将血红蛋白(Arg-31α)的α链的精氨酸残基修饰为氢咪唑啉酮。
结论:乙二醛在糖尿病中的升高水平及其高反应性可能导致血红素蛋白的修饰。因此,考虑到在生理条件下乙二醛诱导的蛋白质修饰的重要性,就了解在体内条件下氢咪唑酮介导的蛋白质修饰而言,该观察结果在临床上显得相关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号