Current Computer-Aided Drug Design ( IF 1.7 ) Pub Date : 2020-07-31 , DOI: 10.2174/1573409915666190710094425 Asmaa M Fahim 1 , Mohamed S Elshikh 2 , Noura M Darwish 3
|
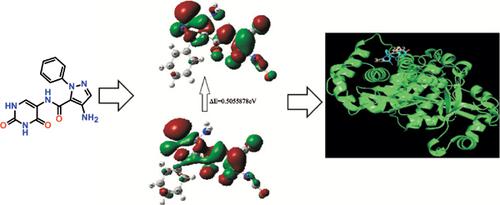
Background: In this investigation, 2-cyano-N-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl) acetamide (3) reacts with dimethylformamide dimethyl acetal (DMF-DMA) to afford the corresponding (E)- 2-cyano-3-(dimethylamino)-N-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)acrylam-ide (4) utilizing microwave irradiation. The condensation reactions of acrylamide derivative 4 with hydrazine derivatives obtain pyrazole derivatives 6a and 6b; respectively. The synthesized compounds demonstrate in vitro antitumor activity against liver tumor cell line HepG2. Furthermore, additional studies were carried out on the most effective compound 6b to evaluate the potential interaction against 4hdq synthase complex with ΔE= -4.5Kcal/mol and with short distance = 1.727Å and 2.027Å, respectively. The comprehensive theoretical studies of compounds 6a and 6b is based on bond length, bond angles and energy gap HOMO-LUMO. In addition, the vibrational frequencies of optimized compounds 6a and 6b were examined through DFT/B3LYP/6+31G(d) basis set.
Methods: In this research, synthesis of novel pyrimidiopyrazole derivatives calculated the computational studies to find suitable drug-receptor interactions and biological activity.
Results and Discussion: The synthesized pyrimidiopyrazole derivative 6b exhibited high antitumor activity IC50 =12.6 μg/ml and interacted it with 4hdq synthase complex with ΔE=-4.5Kcal/mol and with short distance = 1.727Å and 2.027Å. Furthermore, the optimized compounds utilize Gaussian 09W.
Conclusion: In the optimized pyrimidiopyrazole derivatives, 6b showed better antitumor activity HeG-2 against 5-flurouracil due to its energy and confirmed more potent of hydrogen bond interaction with protein pocket.
中文翻译:

新型嘧啶并吡唑衍生物的合成,抗肿瘤活性,分子对接和DFT研究。
背景:在这项研究中,2-氰基-N-(2,4-二氧-1,2,3,4-四氢嘧啶-5-基)乙酰胺(3)与二甲基甲酰胺二甲基乙缩醛(DMF-DMA)反应,得到相应的(E)-2-氰基-3-(二甲基氨基)-N-(2,4-二氧-1,2,3,4-四氢嘧啶-5-基)丙烯酰胺(4)。丙烯酰胺衍生物4与肼衍生物的缩合反应得到吡唑衍生物6a和6b; 分别。合成的化合物显示出对肝肿瘤细胞系HepG2的体外抗肿瘤活性。此外,对最有效的化合物6b进行了进一步的研究,以评估针对4hdq合酶复合物的潜在相互作用,分别具有ΔE= -4.5Kcal / mol和短距离=1.727Å和2.027Å。化合物6a和6b的全面理论研究基于键长,键角和能隙HOMO-LUMO。此外,通过DFT / B3LYP / 6 + 31G(d)基组检查了优化化合物6a和6b的振动频率。
方法:在这项研究中,合成新的嘧啶二吡唑衍生物进行了计算研究,以发现合适的药物-受体相互作用和生物活性。
结果与讨论:合成的嘧啶二吡唑衍生物6b具有较高的抗肿瘤活性,IC50 = 12.6μg/ ml,并与ΔE= -4.5Kcal / mol,短距离=1.727Å和2.027Å的4hdq合酶复合物相互作用。此外,优化的化合物利用高斯09W。
结论:在优化的嘧啶二吡唑衍生物中,6b由于其能量具有更好的HeG-2对5-氟尿嘧啶的抗肿瘤活性,并证实了与蛋白口袋的氢键相互作用更有效。



























 京公网安备 11010802027423号
京公网安备 11010802027423号