Current Pharmaceutical Biotechnology ( IF 2.8 ) Pub Date : 2020-06-30 , DOI: 10.2174/1389201020666190405182251 Gil Bub 1 , Matthew J Daniels 2
|
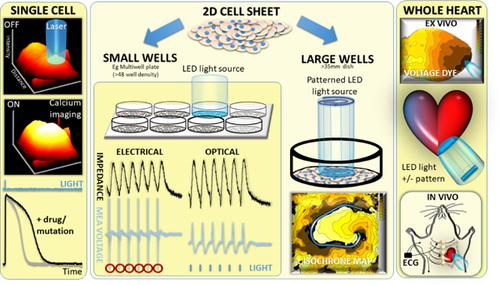
In 1791, Galvani established that electricity activated excitable cells. In the two centuries that followed, electrode stimulation of neuronal, skeletal and cardiac muscle became the adjunctive method of choice in experimental, electrophysiological, and clinical arenas. This approach underpins breakthrough technologies like implantable cardiac pacemakers that we currently take for granted. However, the contact dependence, and field stimulation that electrical depolarization delivers brings inherent limitations to the scope and experimental scale that can be achieved. Many of these were not exposed until reliable in vitro stem-cell derived experimental materials, with genotypes of interest, were produced in the numbers needed for multi-well screening platforms (for toxicity or efficacy studies) or the 2D or 3D tissue surrogates required to study propagation of depolarization within multicellular constructs that mimic clinically relevant arrhythmia in the heart or brain. Here the limitations of classical electrode stimulation are discussed. We describe how these are overcome by optogenetic tools which put electrically excitable cells under the control of light. We discuss how this enables studies in cardiac material from the single cell to the whole heart scale. We review the current commercial platforms that incorporate optogenetic stimulation strategies, and summarize the global literature to date on cardiac applications of optogenetics. We show that the advantages of optogenetic stimulation relevant to iPS-CM based screening include independence from contact, elimination of electrical stimulation artefacts in field potential measuring approaches such as the multi-electrode array, and the ability to print re-entrant patterns of depolarization at will on 2D cardiomyocyte monolayers.
中文翻译:

在心肌细胞表型分析中使用辅助光遗传学技术的可行性 - 从单细胞到整个心脏。
1791年,伽伐尼确立了电可以激活可兴奋细胞。在接下来的两个世纪中,神经元、骨骼和心肌的电极刺激成为实验、电生理学和临床领域首选的辅助方法。这种方法支持了诸如植入式心脏起搏器之类的突破性技术,而我们目前认为这些技术是理所当然的。然而,电去极化所提供的接触依赖性和场刺激给可实现的范围和实验规模带来了固有的限制。其中许多直到具有感兴趣的基因型的可靠的体外干细胞衍生实验材料以多孔筛选平台(用于毒性或功效研究)所需的数量或所需的 2D 或 3D 组织替代物产生后才暴露。研究模拟心脏或大脑中临床相关心律失常的多细胞结构内去极化的传播。这里讨论经典电极刺激的局限性。我们描述了如何通过光遗传学工具克服这些问题,这些工具将电兴奋细胞置于光的控制下。我们讨论这如何能够对从单细胞到整个心脏尺度的心脏材料进行研究。我们回顾了当前结合光遗传学刺激策略的商业平台,并总结了迄今为止关于光遗传学心脏应用的全球文献。我们表明,与基于 iPS-CM 的筛选相关的光遗传学刺激的优点包括独立于接触、消除场电位测量方法(例如多电极阵列)中的电刺激伪影,以及在将在二维心肌细胞单层上。


























 京公网安备 11010802027423号
京公网安备 11010802027423号