Current Computer-Aided Drug Design ( IF 1.7 ) Pub Date : 2020-05-31 , DOI: 10.2174/1573409915666181211114743 Reema A Khalaf 1 , Dalal Masalha 1 , Dima Sabbah 1
|
Background: Lately, diabetes has become the main health concern for millions of people around the world. Dipeptidyl peptidase-IV (DPP-IV) inhibitors have emerged as a new class of oral antidiabetic agents. Formerly, acridines, N4-sulfonamido-succinamic, phthalamic, acrylic and benzoyl acetic acid derivatives, and sulfamoyl-phenyl acid esters were designed and developed as new DPP-IV inhibitors.
Objective: This study aims to develop a pharmacophore model of DPP-IV inhibitors and to evaluate phenanthridines as a novel scaffold for inhibiting DPP-IV enzyme. In addition, to assess their binding interactions with the enzyme through docking in the binding site of 4A5S (PDB).
Methods: Herein, Quantum–Polarized Ligand Docking (QPLD) and ligand-based pharmacophore modeling investigations were performed. Three novel 3,8-disubstituted-6-phenyl phenanthridine derivatives 3-5 have been designed, synthesized and characterized. In vitro biological testing against DPP-IV was carried out using fluorometric assay kit.
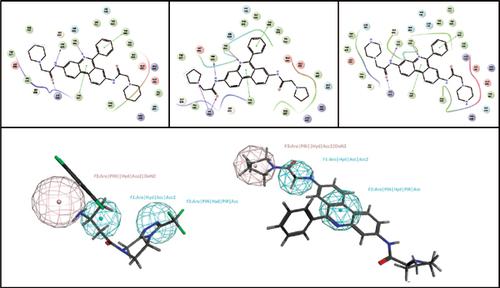
Results: QPLD study demonstrates that compounds 3-5 forms H-bond with Lys554, Trp629, and Tyr631, besides charge transfer interaction between their aromatic rings and the aromatic rings of Tyr547 and Tyr666. Moreover, they fit the three pharmacophoric point features of DPP-IV inhibitors and were proven to have in vitro DPP-IV inhibitory activity where compound 5 displayed a % inhibition of 45.4 at 100 μM concentration.
Conclusion: Phenanthridines may serve as a potential lead compound for developing new DPP-IV inhibitors as a promising antidiabetic agent. Computational results suggest future structural simplification.
中文翻译:

DPP-IV抑制性菲啶:配体,基于结构的设计和合成。
背景:最近,糖尿病已成为全球数百万人关注的主要健康问题。二肽基肽酶-IV(DPP-IV)抑制剂已成为一类新型的口服降糖药。以前,designed啶,N4-磺酰胺基丁二酸,邻苯二甲酸,丙烯酸和苯甲酰乙酸衍生物以及氨磺酰基-苯基酸酯被设计和开发为新型的DPP-IV抑制剂。
目的:本研究旨在建立DPP-IV抑制剂的药效基团模型,并评估菲啶作为抑制DPP-IV酶的新型支架。此外,通过停靠在4A5S(PDB)的结合位点来评估它们与酶的结合相互作用。
方法:本文进行了量子极化配体对接(QPLD)和基于配体的药效团建模研究。已经设计,合成和表征了三种新颖的3,8-二取代的6-苯基菲啶衍生物3-5。使用荧光测定试剂盒对DPP-IV进行了体外生物学测试。
结果:QPLD研究表明,化合物3-5除其芳环与Tyr547和Tyr666的芳环之间的电荷转移相互作用外,还与Lys554,Trp629和Tyr631形成H键。此外,它们符合DPP-IV抑制剂的三个药效学特征,并被证明具有体外DPP-IV抑制活性,其中化合物5在100μM浓度下显示出45.4%的抑制率。
结论:菲啶可能是开发新的DPP-IV抑制剂作为潜在的抗糖尿病药物的潜在先导化合物。计算结果表明未来的结构简化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号