Ferritin‐Nanocaged Aggregation‐Induced Emission Drug Vitexin Inhibits Ferroptosis to Treat Asthenozoospermia
Advanced Functional Materials ( IF 19 ) Pub Date : 2025-10-29 , DOI: 10.1002/adfm.202522020
Xinghua Yu , Yujun Zhang , Lingan Zeng , Haiyan Chen , Xuemei Dong , Tianfu Zhang , Ben Zhong Tang , Fei Sun
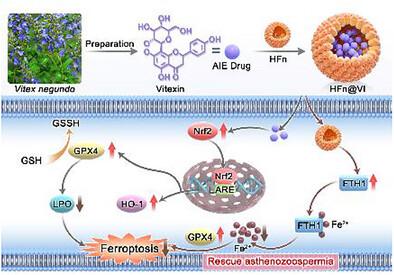 |
Asthenozoospermia, a major cause of male infertility characterized by impaired sperm motility, is critically driven by oxidative stress and dysregulated iron metabolism, which directly induce ferroptosis in sperm cells. To address this, a novel nanotheranostic platform utilizing ferritin nanocages to encapsulate vitexin, a natural flavonoid with potent antioxidant properties and unique aggregation‐induced emission (AIE) characteristics, is introduced. This ferritin nanocage‐loaded vitexin (HFn@VI) system combines the iron‐chelating capability of ferritin with vitexin's ability to mitigate oxidative stress, enabling precise dual‐targeting of the ferroptotic pathways in spermatogenic cells. In the asthenozoospermia model mice, HFn@VI significantly improves sperm count and kinematic parameters (motility, velocity) without observable systemic toxicity, demonstrating superior therapeutic efficacy. Mechanistic studies reveal that the ferritin component elevates ferritin heavy chain 1(FTH1) levels, sequestering free iron ions and reducing cellular iron overload. Concurrently, vitexin activates the nuclear factor erythroid 2‐related factor 2 (Nrf2)/heme oxygenase‐1 (HO‐1) pathway, enhancing glutathione peroxidase 4 (GPX4) expression to inhibit lipid peroxidation and ferroptosis. This study pioneers the application of vitexin, a natural product exhibiting AIE characteristics, as a therapeutic agent in reproductive medicine and underscores the potential of dual‐target nanomedicines for precise ferroptosis intervention, establishing a new paradigm for treating male infertility through advances in material science.
中文翻译:

铁蛋白纳米笼聚集诱导发射药物荆条抑制铁死亡治疗弱精子症
弱精子症是男性不育症的主要原因,其特征是精子活力受损,其关键驱动因素是氧化应激和铁代谢失调,直接诱导精子细胞铁死亡。为了解决这个问题,引入了一种新型纳麦素分析平台,利用铁蛋白纳米笼封装牡荆素,牡荆素是一种具有强大抗氧化特性和独特聚集诱导发射 (AIE) 特性的天然类黄酮。这种载有铁蛋白纳米笼的牡荆素 (HFn@VI) 系统结合了铁蛋白的铁螯合能力和牡荆素减轻氧化应激的能力,能够精确地双靶向生精细胞中的铁通路。在弱精子动物症模型小鼠中,HFn@VI 显着改善精子数量和运动学参数(运动性,速度),而没有观察到的全身毒性,显示出卓越的治疗效果。机理研究表明,铁蛋白成分可提高铁蛋白重链 1 (FTH1) 水平,螯合游离铁离子并减少细胞铁过载。同时,牡荆素激活核因子红细胞 2 相关因子 2 (Nrf2)/血红素加氧酶 1 (HO-1) 通路,增强谷胱甘肽过氧化物酶 4 (GPX4) 的表达,从而抑制脂质过氧化和铁死亡。本研究开创了具有 AIE 特性的天然产物牡荆素作为治疗剂在生殖医学中的应用,强调了双靶点纳米药物在铁死亡精准干预方面的潜力,通过材料科学的进步为治疗男性不育症建立了新的范式。
Blood-testis barrier-crossing extracellular vesicles for asthenozoospermia therapy via synergistic ATP replenishment and ferroptosis suppression
Biomaterials ( IF 12.9 ) Pub Date : 2025-10-13 , DOI: 10.1016/j.biomaterials.2025.123777
Xinghua Yu , Lingan Zeng , Haiyan Chen , Xuemei Dong , Fan Wen , Mingming Wang , Runqi Pan , Yujun Zhang , Wei Zhu , Dingyuan Yan , Dong Wang , Fei Sun
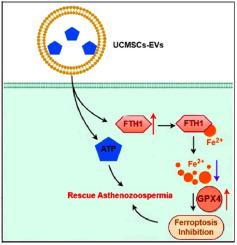 |
Asthenozoospermia, a leading cause of male infertility characterized by impaired sperm motility, poses significant therapeutic challenges due to the restrictive blood-testis barrier (BTB). To address this limitation, we engineered multifunctional extracellular vesicles (EVs) derived from umbilical cord mesenchymal stem cells, loaded with adenosine triphosphate (ATP) to synergistically restore cellular bioenergetics and suppress ferroptosis. These EVs were further functionalized with an aggregation-induced emission luminogen for near-infrared-II (NIR-II) fluorescence imaging, enabling real-time visualization of their efficient BTB penetration and targeted accumulation within testicular tissues. Upon localization in the seminiferous tubules, the released ATP directly replenishes the energy reserves of spermatogenic cells and enhances sperm motility in asthenozoospermia models. Simultaneously, the EVs upregulate glutathione peroxidase 4, mitigating ferroptosis and synergizing with ATP to restore metabolic homeostasis. In vivo studies demonstrate the dual efficacy of this platform: the EVs precisely traverse the BTB while robustly inhibiting ferroptosis without systemic toxicity, significantly improving sperm count and kinematic parameters (motility, velocity). This study presents a multifunctional nanoplatform that integrates NIR–II–guided imaging, ATP-mediated energy restoration, and pathology-specific ferroptosis inhibition, providing a promising non-invasive therapeutic strategy for asthenozoospermia.
An NIR‐II Absorbing Injectable Hydrogel for Boosted Photo‐Immunotherapy Toward Human Papillomavirus Associated Cancer
Aggregate ( IF 13.9 ) Pub Date : 2025-01-21 , DOI: 10.1002/agt2.743
Xinghua Yu 1 , Lingan Zeng 1 , Xinyue Yang 2 , Zuliang Ren 1 , Xuemei Dong 1 , Ge Meng 1 , Guogang Shan 2 , Dingyuan Yan 3 , Dong Wang 3 , Fei Sun 1
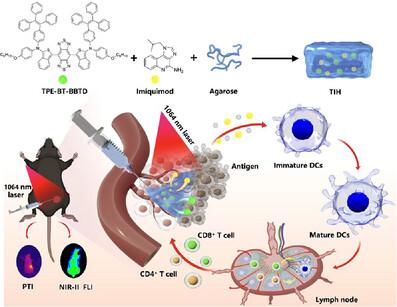 |
Human papillomavirus (HPV) is a highly prevalent venereal pathogen accounting for genital warts and various cancers like cervical, anal, and oropharyngeal cancers. Although imiquimod, a topical medication, is commonly used to treat genital warts induced by HPV, its potential as an in situ immune response regulator for HPV‐related cancers has rarely been explored. In this study, we developed an innovative synergistic therapeutic platform by integrating near‐infrared‐II (NIR‐II) absorbing aggregation‐induced emission (AIE) agent (TPE‐BT‐BBTD) and imiquimod into an injectable hydrogel named TIH. TPE‐BT‐BBTD molecule that serves as a photothermal agent, with exposure to a 1064 nm laser, effectively destroys tumor cells and releases tumor‐related antigens. During the thermogenesis process, the hydrogel melts and releases imiquimod. The released imiquimod, in conjunction with the dead tumor antigens, stimulates dendritic cell maturation, activating the immune system to ultimately eliminate residual cancer cells. This novel approach combines the immunomodulatory effects of imiquimod with a 1064 nm‐excitable photothermal agent in a hydrogel delivery system, offering a promising tactic for combating HPV‐associated cancers.
A dual-modality hydrogen sulfide-specific probe integrating chemiluminescence with NIR fluorescence for targeted cancer imaging
Science China Chemistry ( IF 10.4 ) Pub Date : 2023-05-05 , DOI: 10.1007/s11426-023-1579-y
Xuemei Dong , Lixin Sun , Ziwen Zhang , Tianli Zhu , Jie Sun , Jinzhu Gao , Chengjun Dong , Rongchen Wang , Xianfeng Gu , Chunchang Zhao
Taking apart in numerous physiological and pathological activities, hydrogen sulfide (H2S) has been selected as an excellent target spot for the early diagnosis of cancer. So far, there are many mature probes that apply single optical imaging to monitor endogenous H2S. Nevertheless, a single modality is not an ideal method to afford accurate diagnostic information in comprehensive biological organisms. Herein, we developed a dual-modal imaging probe BWS. This designed probe showed a specific response to H2S with both chemiluminescence and NIR fluorescence light-up. The concurrence of fluorescence and chemiluminescence signal provided “double insurances” for highly accurate monitoring of H2S. Satisfactorily, this dual-modal imaging probe performed precise visualization of endogenous H2S in living cells and in vivo. We envisaged that this chemiluminescence/fluorescence real-time dual-modality strategy for H2S detection will expand and optimize the multimodal imaging methods for efficient diagnosis and treatment of cancer.
RSS and ROS Sequentially Activated Carbon Monoxide Release for Boosting NIR Imaging-Guided On-Demand Photodynamic Therapy
Small ( IF 13.0 ) Pub Date : 2023-12-15 , DOI: 10.1002/smll.202309529
Xuemei Dong 1 , Ziwen Zhang 2 , Rongchen Wang 1 , Jie Sun 1 , Chengjun Dong 1 , Lixin Sun 1 , Cai Jia 3 , Xianfeng Gu 2 , Chunchang Zhao 1
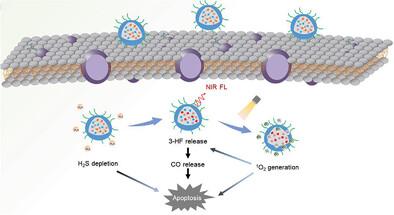
Carbon monoxide shows great therapeutic potential in anti-cancer. In particular, the construction of multifunctional CO delivery systems can promote the precise delivery of CO and achieve ideal therapeutic effects, but there are still great challenges in design. In this work, a RSS and ROS sequentially activated CO delivery system is developed for boosting NIR imaging-guided on-demand photodynamic therapy. This designed system is composed of a CO releaser (BOD-CO) and a photosensitizer (BOD-I). BOD-CO can be specifically activated by hydrogen sulfide with simultaneous release of CO donor and NIR fluorescence that can identify H2S-rich tumors and guide light therapy, also depleting H2S in the process. Moreover, BOD-I generates 1O2 under long-wavelength light irradiation, enabling both PDT and precise local release of CO via a photooxidation mechanism. Such sequential activation of CO release by RSS and ROS ensured the safety and controllability of CO delivery, and effectively avoided leakage during delivery. Importantly, cytotoxicity and in vivo studies reveal that the release of CO combined with the depletion of endogenous H2S amplified PDT, achieving ideal anticancer results. It is believed that such theranostic nanoplatform can provide a novel strategy for the precise CO delivery and combined therapy involved in gas therapy and PDT.
Depletion and Downregulation of Hydrogen Sulfide Using an Activatable Probe for Promoting Photothermal Therapy toward Colorectal Cancers
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-04-25 , DOI: 10.1021/acs.jmedchem.4c00264
Xuemei Dong 1 , Hongyu Wu 2 , Ziwen Zhang 2 , Jie Sun 1 , Chengjun Dong 1 , Lixin Sun 1 , Rongchen Wang 1 , Xianfeng Gu 2 , Chunchang Zhao 1
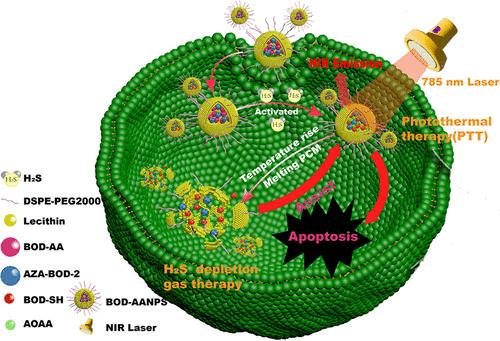 |
Since hydrogen sulfide (H2S) is an important endogenous gaseous mediator, therapeutic manipulation of H2S is promising for anticancer treatment. In this work, we develop a novel theranostic nanoplatform with H2S-specific and photocontrolled synergistic activation for imaging-guided H2S depletion and downregulation along with promoted photothermal therapy. Such a nanoplatform is fabricated by integration of a H2S-responsive molecule probe that can generate a cystathionine-β-synthase (CBS) inhibitor AOAA and a photothermal transducer into an NIR-light-responsive container. Our nanoplatform can turn on NIR fluorescence specifically in H2S-rich cancers, guiding further laser irradiation. Furthermore, prominent conversion of photoenergy into heat guarantees special container melting with controllable AOAA release for H2S-level downregulation. This smart regulation of the endogenous H2S level amplifies the PTT therapeutic effect, successfully suppressing colorectal tumor in living mice under NIR fluorescence imaging guidance. Thus, we believe that this nanoplatform may provide a powerful tool toward H2S-concerned cancer treatment with an optimized diagnostic and therapeutic effect.
Specific imaging of intracellular hydrogen sulfide by a positively charged NIR fluorescent probe
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2023-10-02 , DOI: 10.1016/j.bmcl.2023.129495
Jianjun Fang 1 , Xuemei Dong 1 , Lixin Sun 1 , Jie Sun 1 , Chengjun Dong 1 , Rongchen Wang 1 , Chunchang Zhao 1
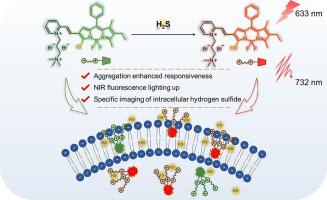
The poor water solubility of traditional activatable organic molecular probes usually limits their detection ability in physiological environment. In this work, a positively charged H2S probe was designed, which exhibited a significantly enhanced responsiveness to H2S in the aggregated state due to the increased positive charge density on the aggregate surface. Under physiological conditions, the probe could be activated by H2S with specificity and sensitivity to release near-infrared fluorescence signal. Moreover, endogenous H2S levels in living cells were successfully monitored by using this probe. We expect that this probe can provide a new strategy for the design of activatable probes to break the limitation of poor water solubility of conventional organic molecular probes.