Published on: 21st January 2021
文章链接:https://pubs.rsc.org/en/content/articlelanding/2021/ta/d0ta09607e#!divRelatedContent&articles
Utilization of hydrocarbons emit a large amount of CO2, while crude oil as the conventional source of hydrocarbons will eventually be depleted. These problems can be potentially solved via a bifunctional catalytic system that can convert CO2 into hydrocarbons.
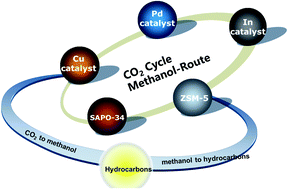
There are two possible reaction pathways involved in this catalytic system, but as compared to the Reverse Water Gas Shift-Fisher Tropsch Synthesis (RWGS-FTS) route, Methanol route potentially has higher desired products selectivity due to its ability to overcome the restriction of Anderson-Schulz-Flory (ASF) distribution. Methanol route requires bifunctional catalysts consisting of metal/metal oxide catalyst and zeolite component. The former is used to convert CO2 into methanol, while the latter is used to convert methanol into hydrocarbons.
In this review, the development of metal/metal oxide (Cu, Pd and In-based catalysts) single component catalyst to enhance CO2 hydrogenation, methanol selectivity, and yield are discussed first. Next, the development of zeolite single component catalyst (ZSM-5 and SAPO-34) for methanol to hydrocarbon (MTH) reaction by minimizing coke formation and tuning hydrocarbon selectivity is discussed. Finally, the development of integrated bifunctional catalysts components (meta/metal oxide-zeolite) via methanol route is introduced. The rational design of single component and integrated components are also compiled.
Overall, while the bifunctional catalytic system is able to successfully achieve the desired product selectivity in all hydrocarbons, it suffers from high CO selectivity at the high operating temperature. Hence, addressing this problem is essential to enhance the yield and selectivity of a specific hydrocarbon. At this stage, it could not be confirmed if the bifunctional catalytic system can effectively decrease CO2 content globally while playing a role as an effective alternative hydrocarbons source until a complete Life Cycle Analysis is being conducted on it.
Nevertheless, it is concluded that bifunctional catalytic system has demonstrated much better potential and benefits than separate multi-steps catalysts in different reactors. This is because, at the same reaction conditions, bifunctional catalysts demonstrate higher CO2 conversion and lower CO selectivity than single metal/metal oxide catalyst, and longer catalyst lifetime than single zeolite catalyst while maintaining the selectivity of desired hydrocarbons product.