Abstract
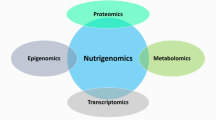
Lifestyle optimizations are implementable changes that can have an impact on health and disease. Nutrition is a lifestyle optimization that has been shown to be of great importance in cancer initiation, progression, and metastasis. Dozens of clinical trials are currently in progress that focus on the nutritional modifications that cancer patients can make prior to and during medical care that increase the efficacy of treatment. In this review, we discuss various nutritional inventions for cancer patients and the analytical approaches to characterize the downstream molecular effects. We first begin by briefly explaining the many different forms of nutritional intervention currently being used in cancer treatment as well as their motivating biology. The forms of nutrient modulation described in this review include calorie restriction, the different practices of fasting, and carbohydrate restriction. The review then shifts to explain how proteomics is used to determine biomarkers of cancer and how it can be utilized in the future to determine the metabolic phenotype of a tumor, and inform physicians if nutritional intervention should be recommended for a cancer patient. Nutrigenomics aims to understand the relationship of nutrients and gene expression and can be used to understand the downstream molecular effects of nutrition restriction, partially through proteomic analysis. Proteomics is just beginning to be used as cancer diagnostic and predictive tools. However, these approaches have not been used to their full potential to understand nutritional intervention in cancer.

ᅟ




Similar content being viewed by others
Abbreviations
- 3D:
-
Three-dimensional
- ASAP2 :
-
A scalable automated proteomic pipeline
- CORE:
-
Cellular consumption and release
- CR:
-
Calorie restriction
- CRP:
-
c-Reactive protein
- CRS:
-
Caloric Restriction Society
- DSR:
-
Differential stress resistance
- ELISA:
-
Enzyme-linked immunosorbent assays
- ESI-MS/MS:
-
Electrospray-ionization tandem mass spectrometry
- FT-ICR:
-
Fourier transform-ion cyclotron resonance
- GH:
-
Growth hormone
- Hba1c:
-
Hemoglobin A1C
- IF:
-
Intermittent fasting
- IGF-1:
-
Insulin growth factor-1
- JAK/STAT:
-
Janus kinase and signal transducer activator of transcription
- KD:
-
Ketogenic diet
- LC:
-
Liquid chromatography
- MRM:
-
Multiple reaction monitoring
- MS:
-
Mass spectrometry
- NF-κB:
-
Nuclear factor-κB
- NIA:
-
National Institute of Aging
- PASEF:
-
Parallel accumulation serial fragmentation
- SDS:
-
Sodium dodecyl sulfate
- S-Traps:
-
Suspension trapping
- TOF:
-
Time-of-flight
- TRF:
-
Time-restricted feeding
- UW:
-
University of Wisconsin, Madison
References
Donaldson MS. Nutrition and cancer: a review of the evidence for an anti-cancer diet. Nutrition. 2004;3:19.
Calle EE, Rodriguez C, Walker-thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a propsectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38.
Lv M, Zhu X, Wang H, Wang F, Guan W. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of Cancer in animal models: a systematic review and meta-analysis. PLoS One. 2014;9:e115147.
Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91.
Samraj AN, Pearce OMT, Läubli H, Crittenden AN, Bergfeld AK, Banda K, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci. 2015;112:542 LP–547.
Kannen V, Zanette DL, Fernandes CR, Ferreira FR, Marini T, Carvalho MC, et al. High-fat diet causes an imbalance in the colonic serotonergic system promoting adipose tissue enlargement and dysplasia in rats. Toxicol Lett. 2012;213:135–41.
Chang H-H, Moro A, Takakura K, Su H-Y, Mo A, Nakanishi M, et al. Incidence of pancreatic cancer is dramatically increased by a high fat, high calorie diet in KrasG12D mice. PLoS One. 2017;12:e0184455.
Cangemi A, Fanale D, Rinaldi G, Bazan V, Galvano A, Perez A, et al. Dietary restriction: could it be considered as speed bump on tumor progression road? Tumor Biol. 2016:1–10.
Simone BA, Champ CE, Rosenberg AL, Berger AC, Monti DA, Dicker AP, et al. Selectively starving cancer cells through dietary manipulation: methods and clinical implications. Future Oncol. 2013;9:959.
Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–12.
McCay CM, Crowell MF, Maynakd LA. The effect of retarded growth upon the length of life span and upon ultimate body size. J Nutr. 1935:63–79.
Weinfruch R, Sohal RS. Caloric intake and aging. N Engl J Med. 1997;337:986–94.
Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–21.
Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4.
Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:1–12.
Renault B, Remond A. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–8.
Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E. Approaches for quantifying energy intake and % calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. Am J Physiol Endocrinol Metab. 2012;302:E441–8.
Kristan DM. Calorie restriction and susceptibility to intact pathogens. Age (Dordr). 2008:147–56.
Roth GS, Kowatch MA, Hengemihle J, Ingram DK, Spangler EL, Johnson LK, et al. Effect of age and caloric restriction on cutaneous wound closure in rats and monkeys. J Gerontol. 1997;52:98–102.
Reed MJ, Pennb PE, Lib Y, Birnbaum R, Vernon RB, Johnsonb TS, et al. Enhanced cell proliferation and biosynthesis mediate improved wound repair in refed , caloric-restricted mice. Mech Ageing Dev. 1996:21–43.
Raffaghello L, Safdie F, Bianchi G, Dorff T, Longo VD. Fasting and differential chemotherapy protection in patients. Cell Cycle. 2010;9:4474–6.
Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–92.
Rouhani MH, Azadbakht LI. Ramadan fasting related to health outcomes? A review on the related evidence. J Res Med Sci. 2014;19:987–92.
Omiecinski CJ, Vanden Heuvel JP, Perdew GH, Peters JM. Xenobiotic metabolism, disposition, and regulation by receptors: from biochemical phenomenon to predictors of major toxicities. Toxicol Sci. 2011;120:S49–75.
Brandhorst S, Wei M, Hwang S, Morgan TE, Longo VD. Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp Gerontol. 2013;48:1120–8.
Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, et al. Fasting and cancer treatment in humans: a case series report. Aging (Albany NY). 2009;1:988–1007.
Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci. 2008;105:8215–20.
Longo VD, Ellerby LM, Bredesen DE, Valentine JS, Gralla EB. Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J Cell Biol. 1997;137:1581–8.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Lee C, Raffaghello L, Longo VD. Starvation, detoxification, and multidrug resistance in cancer therapy. Drug Resist Updat. 2012;15:114–22.
Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–16.
K a V, Roohk DJ, Hellerstein MK. Dose effects of modified alternate-day fasting regimens on in vivo cell proliferation and plasma insulin-like growth factor-1 in mice. J Appl Physiol. 2007;103:547–51.
Varady KA, Roohk DJ, Bruss M, Hellerstein MK. Alternate-day fasting reduces global cell proliferation rates independently of dietary fat content in mice. Nutrition. 2009;25:486–91.
Johnson JB, John S, Laub DR. Pretreatment with alternate day modified fast will permit higher dose and frequency of cancer chemotherapy and better cure rates. Med Hypotheses. 2009;72:381–2.
Varady KA, Roohk DJ, McEvoy-Hein BK, Gaylinn BD, Thorner MO, Hellerstein MK. Modified alternate-day fasting regimens reduce cell proliferation rates to a similar extent as daily calorie restriction in mice. FASEB J. 2008;22:2090–6.
Van NG, Hattingh SM, Engelbrecht A. Enhanced therapeutic efficacy in cancer patients by short-term fasting: the autophagy connection. Front Oncol. 2016;6:1–7.
Kozubík A, Pospísil M. Protective effect of intermittent fasting on the mortality of gamma-irradiated mice. Strahlentherapie. 1982;152:734–8.
Descamps O, Riondel J, Ducros V, Roussel A. Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting. Mech Ageing Dev. 2005;126:1185–91.
Cleary M, Grossmann M. The manner in which calories are restricted impacts mammary tumor cancer prevention. J Carcinog. 2011;10:21.
Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–37.
Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, et al. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem. 2010;21:413–7.
Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Differential effects of intermittent feeding and voluntary exercise on body weight and lifespan in adult rats. J Gerontol. 1983;38:36–45.
Rothschild J, Hoddy KK, Jambazian P, Varady KA. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. 2014;72:308–18.
Manoogian ENC, Panda S. E.N.C. M. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res Rev. 2016;39:59–67.
Hatori M, Vollmers C, Zarrinpar A, Ditacchio L, Bushong EA, Gill S. Time restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high fat diet. Cell Metab. 2012;15:848–60.
Hayashi Y, Ushijima K, Ando H, Yanagihara H, Ishikawa E, Tsuruoka S-I. Influence of a time-restricted feeding schedule on the daily rhythm of abcb1a gene expression and its function in rat intestine. J Pharmacol Exp Ther. 2010;335:418–23.
Melkani GC, Panda S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J Physiol. 2017;595:3961–700.
Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005.
Marinac CR, Sears DD, Natarajan L, Gallo LC, Breen CI, Patterson RE. Frequency and circadian timing of eating may influence biomarkers of inflammation and insulin resistance associated with breast cancer risk. PLoS One. 2015;10:1–11.
Marinac CR, Natarajan L, Sears DD, Gallo LC, Hartman SJ, Arredondo E. Prolonged nightly fasting and breast cancer risk: findings from NHANES (2009-2010). Cancer Epidemiol Biomark Prev. 2015;24:783–9.
Hite AH, Berkowitz VG, Berkowitz K. Low-carbohydrate diet review: shifting the paradigm. Nutr Clin Pract. 2011;26:300–8.
Bozzetti F, Mori V. Nutritional support and tumour growth in humans: a narrative review of the literature. Clin Nutr. 2009;28:226–30.
Schroll MM, Liu X, Herzog SK, Skube SB, Hummon AB. Nutrient restriction of glucose or serum results in similar proteomic expression changes in 3D Colon cancer cell cultures. Nutr Res. 2016;36:1068–80.
Zhou W, Mukherjee P, Kiebish MA, Mantis JG, Gorham KN, Mulrooney TJ. Ketocal®, a novel ketogenic diet therapy for brain cancer. Proc Am Assoc Cancer Res. 2006;47:914.
Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet , an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:1–15.
Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res. 2013;19:3905–13.
Mavropoulos JC, Buschemeyer WC 3rd, Tewari a K, Rokhfeld D, Pollak M, Zhao Y. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev Res. 2009;2:557–65.
Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, et al. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer. 2008;8:122.
Dang MT, Wehrli S, Dang CV, Curran T. The ketogenic diet does not affect growth of hedgehog pathway medulloblastoma in mice. PLoS One. 2015;10
Tan-Shalaby JL, Carrick J, Edinger K, Genovese D, Liman AD, Passero VA, et al. Modified Atkins diet in advanced malignancies - final results of a safety and feasibility trial within the veterans affairs Pittsburgh healthcare system. Nutr Metab. 2016;13:52.
Mathews EH, Liebenberg L. Short-term starvation for cancer control in humans. Exp Gerontol. 2013;48:1293.
Saudek CD. The metabolic events of starvation. Am J Med. 1976;60:117–26.
Wang T, Hung CCY, Randall DJ. The comparative physiology of rood deprivation: from feast to famine. Annu Rev Physiol. 2006;68:223–51.
Méquinion M, Langlet F, Zgheib S, Dickson S, Dehouck B, Chauveau C, et al. Ghrelin: central and peripheral implications in anorexia nervosa. Front Endocrinol. 2013;26:15.
Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;13:89–98.
Florey O, Overholtzer M. Autophagy proteins in macroendocytic engulfment. Trends Cell Biol. 2012;22:374–80.
Panieri E, Toietta G, Mele M, Labate V, Ranieri SC, Fusco S, et al. Nutrient withdrawal rescues growth factor-deprived cells from mTOR-dependent damage. Aging. 2010;2:487–503.
Yu H, Rohan T. Role of the insulin-like growth factor family in Cancer development and progression. J Natl Cancer Inst. 2000;92:1472–89.
Davison Z, De Blacquière GE, Westley BR, FEB M. Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: implications for therapy 1. Neoplasia. 2011;13:504–15.
Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–94.
Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–5.
Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, Palin MF. Adiponectin action from head to toe. Endocrine. 2010;37:11–32.
Otani K, Ishihara S, Yamaguchi H, Murono K, Yasuda K, Nishikawa T, et al. Adiponectin and colorectal cancer. Surg Today. 2017;47:151–8.
Hebbard L, Ranscht B. Multifaceted roles of adiponectin in cancer. Best Pract Res Clin Endocrinol Metab. 2014;28:59–69.
Byeon JS, Jeong JY, Kim MJ, Lee SM, Nam WH, Myung SJ, et al. Adiponectin and adiponectin receptor in relation to colorectal cancer progression. Int J Cancer. 2010;127:2758–67.
Jardé T, Perrier S, Vasson M-P, Caldefie-Chézet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer. 2011;47:33–43.
Hursting SD, Dunlap SM, N a F, Hursting MJ, Lashinger LM. Calorie restriction and cancer prevention: a mechanistic perspective. Cancer Metab. 2013;1:10.
Wolf I, Sadetzki S, Kanely H, Kundel Y, Pariente C, Epstein N, et al. Adiponectin, ghrelin, and leptin in cancer cachexia in breast and colon cancer patients. Cancer. 2006;106:966–73.
Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–4.
Stattin P, Lukanova A, Biessy C, Söderberg S, Palmqvist R, Kaaks R, et al. Obesity and colon cancer: does leptin provide a link? Int J Cancer. 2004;109:149–52.
Surmacz E. Leptin and adiponectin: emerging therapeutic targets in breast cancer. J Mammary Gland Biol Neoplasia. 2013;18:321–32.
Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;1:12–22.
Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47.
Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271–80.
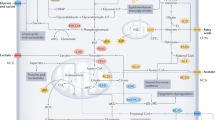
Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–4.
Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–8.
Immanuel SRC, Ghanate AD, Parmar DS, Marriage F, Panchagnula V, Day PJ, et al. Integrative analysis of rewired central metabolism in temozolomide resistant cells. Biochem Biophys Res Commun. 2017;495:2010–6.
Müller M, Kersten S. Nutrigenomics : goals and strategies. Nat Rev Genet. 2003;4:315–22.
Peregrin T. The new frontier of nutrition science: nutrigenomics. J Am Diet Assoc. 2001;101:1306.
Norheim F, Gjelstad IMF, Hjorth M, Vinknes KJ, Langleite TM, Holen T, et al. Molecular nutrition research-the modern way of performing nutritional science. Nutrients. 2012;4:1898–944.
Schweigert FJ. Nutritional proteomics: methods and concepts for research in nutritional science. Ann Nutr Metab. 2007;51:99–107.
Sales NMR, Pelegrini PB, Goersch MC. Nutrigenomics: definitions and advances of this new science. J Nutr Metab. 2014;2014:202759.
Wang J, Li D, Dangott LJ. Wu G. Recent advances in nutritional sciences proteomics and its role in. J Chromatogr. 2006:1759–62.
Afman L, Müller M. Nutrigenomics : from molecular nutrition to prevention of disease. J Am Diet Assoc. 2006:569–76.
Samatov TR, Galatenko VV, Block A, Shkurnikov MY, Tonevitsky AG, Schumacher U. Novel biomarkers in cancer: the whole is greater than the sum of its parts. Semin Cancer Biol. 2017;45:50–7.
Kang JX. Identification of metabolic biomarkers for personalized nutrition. J Nutrigenet Nutrigenomics. 2012;5:I–II.
Ikizler TA. Using and interpreting serum albumin and prealbumin as nutritional markers in patients on chronic dialysis. Semin Dial. 2014;27:590–2.
Pande S, Kratasyuk VA, Medvedeva NN, Kolenchukova OA, Salmina AB. Nutritional biomarkers: current view and future perspectives. Crit Rev Food Sci Nutr. 2017;0:1–15.
Marcason W. Should albumin and prealbumin be used as indicators for malnutrition? J Acad Nutr Diet. 2017;117:1144.
Peveler WJ, Yazdani M, Rotello VM. Selectivity and specificity: pros and cons in sensing. ACS Sensors. 2016;1:1282–5.
Hyldgaard J, Bor P, Ingerslev HJ, Tørring N. Comparison of two different methods for measuring anti-mullerian hormone in a clinical series. Reprod Biol Endocrinol. 2015;13:107.
McDonald WH, Yates JR. Shotgun proteomics and biomarker discovery. Dis Markers. 2002;18:99–105.
Ong S-E, Mann M. Mass spectrometry-based proteomics turns quantitative. Nat Chem Biol. 2005;1:252–62.
Zhang Y, Fonslow BR, Shan B, Baek MC, Yates JR 3rd. Protein analysis by shotgun/bottom-up proteomics. Chem Rev. 2013;113:2343–94.
Sun L, Bertke MM, Champion MM, Zhu G, Huber PW, Dovichi NJ. Quantitative proteomics of Xenopus laevis embryos: expression kinetics of nearly 4000 proteins during early development. Sci Rep. 2014;4:4365.
Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p. p. b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–72.
Wiese S, Reidegeld KA, Meyer HE, Warscheid B. Protein labeling by iTRAQ: a new tool for quantitative mass spectrometry in proteome research. Proteomics. 2007;7:340–50.
Wu WW, Wang G, Baek SJ, Shen RF. Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D gel- or LC-MALDI TOF/TOF. J Proteome Res. 2006;5:651–8.
Li H, Han J, Pan J, Liu T, Parker CE, Borchers CH. Current trends in quantitative proteomics—an update. J Mass Spectrom. 2017;52:319–41.
Wasinger VC, Zeng M, Yau Y. Current status and advances in quantitative proteomic mass spectrometry. Int J Proteomics. 2013;2013:1–12.
Bantscheff M, Lemeer S, Savitski MM, Kuster B. Quantitative mass spectrometry in proteomics: critical review update from 2007 to the present. Anal Bioanal Chem. 2012;404:939–65.
Toby TK, Fornelli L, Kelleher NL. Progress in top-down proteomics and the analysis of proteoforms. Annu Rev Anal Chem. 2016;9:499–519.
Han X, Wang Y, Aslanian A, Fonslow B, Graczyk B, Davis TN, et al. In-line separation by capillary electrophoresis prior to analysis by top-down mass spectrometry enables sensitive characterization of protein complexes. J Proteome Res. 2014;13:6078–86.
Tran JC, Zamdborg L, Ahlf DR, Lee JE, Catherman AD, Durbin KR, et al. Mapping intact protein isoforms in discovery mode using top-down proteomics. Nature. 2011;480:254–8.
McCool E, Lubeckyj RA, Shen X, Chen D, Kou Q, Liu X, et al. Deep top-down proteomics using capillary zone electrophoresis-tandem mass spectrometry: identification of 5700 proteoforms from the Escherichia coli proteome. Anal Chem. 2018;90:5529–33. https://doi.org/10.1021/acs.analchem.8b00693.
Mann M, Kulak NA, Nagaraj N, Cox J. The coming age of complete, accurate, and ubiquitous proteomes. Mol Cell. 2013;49:583–90.
Glatter T, Ahrné E, Schmidt A. Comparison of different sample preparation protocols reveals lysis buffer-specific extraction biases in gram-negative bacteria and human cells. J Proteome Res. 2015;14:4472–85.
Zougman A, Selby PJ, Banks RE. Suspension trapping (STrap) sample preparation method for bottom-up proteomics analysis. Proteomics. 2014;14:1006–0.
Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359.
Geyer PE, Kulak NA, Pichler G, Holdt LM, Teupser D, Mann M. Plasma proteome profiling to assess human health and disease. Cell Syst. 2016;2:185–95.
Beasley-Green A. Urine proteomics in the era of mass spectrometry. Int Neurourol J. 2016;20:S70–5.
Weston LA, Bauer KM, Hummon AB. Comparison of bottom-up proteomic approaches for LC-MS analysis of complex proteomes. Anal Methods. 2013;5 https://doi.org/10.1039/C3AY40853A.
Cao Z, Tang H-Y, Wang H, Liu Q, Speicher DW. Systematic comparison of fractionation methods for in-depth analysis of plasma proteomes. J Proteome Res. 2012;11:3090–100.
Yeung Y-G, Nieves E, Angeletti R, Stanley ER. Removal of detergents from protein digests for mass spectrometry analysis. Anal Biochem. 2008;382:135–7.
Eliuk S, Makarov A. Evolution of Orbitrap mass spectrometry instrumentation. Annu Rev Anal Chem. 2015;8:61–80.
Rousu T, Herttuainen J, Tolonen A. Comparison of triple quadrupole, hybrid linear ion trap triple quadrupole, time-of-flight and LTQ-orbitrap mass spectrometers in drug discovery phase metabolite screening and identification in vitro—amitriptyline and verapamil as model compounds. Rapid Commun Mass Spectrom. 2010;24:939–57.
Meier F, Beck S, Grassl N, Lubeck M, Park MA, Raether O, et al. Parallel accumulation–serial fragmentation (PASEF): multiplying sequencing speed and sensitivity by synchronized scans in a trapped ion mobility device. J Proteome Res. 2015;14:5378–87.
Dayon L, Núñez Galindo A, Cominetti O, Corthésy J, Kussmann MA. Highly automated shotgun proteomic workflow: clinical scale and robustness for biomarker discovery in blood. Serum/plasma. Proteomics. 2017;1619:433–49.
Cieślik M, Chinnaiyan AM. Cancer transcriptome profiling at the juncture of clinical translation. Nat Rev Genet. 2018;19:93–109.
Bausch-Fluck D, Hofmann A, Bock T, Frei AP, Cerciello F, Jacobs A, et al. A mass spectrometric-derived cell surface protein atlas. PLoS One. 2015;10:e0121314.
Huang R, Chen Z, He L, He N, Xi Z, Li Z, et al. Mass spectrometry-assisted gel-based proteomics in cancer biomarker discovery: approaches and application. Theranostics. 2017;7:3559–72.
Borràs E, Sabidó E. What is targeted proteomics? A concise revision of targeted acquisition and targeted data analysis in mass spectrometry. Proteomics. 2017;17:17–8.
Procházková I, Lenčo J, Fučíková A, Dresler J, Čápková L, Hrstka R, et al. Targeted proteomics driven verification of biomarker candidates associated with breast cancer aggressiveness. Biochim Biophys Acta. 2017;1865:488–98.
Shiromizu T, Kume H, Ishida M, Adachi J, Kano M, Matsubara H, et al. Quantitation of putative colorectal cancer biomarker candidates in serum extracellular vesicles by targeted proteomics. Sci Rep. 2017;7:1–13.
Sjöström M, Ossola R, Breslin T, Rinner O, Malmström L, Schmidt A, et al. A combined shotgun and targeted mass spectrometry strategy for breast cancer biomarker discovery. J Proteome Res. 2015;14:2807–18.
Kawashima Y, Singh A, Kodera Y, Matsumoto H. Nutritional proteomics : investigating molecular mechanisms underlying the health beneficial effect of functional foods. Funct Foods Heal Dis. 2013;3:300–9.
Schroll MM, LaBonia GJ, Ludwig KR, Hummon AB. Glucose restriction combined with autophagy inhibition and chemotherapy in HCT 116 spheroids decreases cell Clonogenicity and viability regulated by tumor suppressor genes. J Proteome Res. 2017;16:3009–18.
Pierobon M, Wulfkuhle J, Liotta L, Petricoin E. Application of molecular technologies for phosphoproteomic analysis of clinical samples. Oncogene. 2014;34:805.
Fuchs D, Winkelmann I, Johnson IT, Mariman E, Wenzel U, Daniel H. Proteomics in nutrition research: principles, technologies and applications. Br J Nutr. 2005;94:302–14.
Romagnolo DF, Milner JA. Opportunities and challenges for nutrional proteomics in cancer prevention. J Nutr. 2012;142:225–9.
Acknowledgments
MMS was supported by the National Institutes of Health Training Grant–Chemistry Biochemistry Biology Interface Program (T32GM075762). ABH was supported by the National Institutes of Health (R01GM110406), and the National Science Foundation (CAREER Award, CHE-1351595). MMS researched available literature and was the major contributing author. All authors read and approved the final manuscript. We gratefully acknowledge the assistance of Dr. Susan Skube, Katelyn Ludwig, and Emily Herring for their edits.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Schroll, M.M., Hummon, A.B. Employing proteomics to understand the effects of nutritional intervention in cancer treatment. Anal Bioanal Chem 410, 6371–6386 (2018). https://doi.org/10.1007/s00216-018-1219-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1219-z