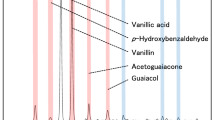
Abstract
The preparation of vanillin from lignin is one of the lignin valorization strategies. However, obtaining high vanillin yield is still a challenge. Therefore, the process of vanillin production and factors that affect yield of vanillin has attracted much attention. Here, oxidation of vanillin was performed to study its degradation behavior under lignin alkaline oxidation conditions. High-performance liquid chromatography, liquid chromatography–electrospray mass spectrometry, gas chromatography–mass spectrometer and gel permeation chromatography were employed to analyze the products including monomers and dimers. Results demonstrated that reaction temperature and time greatly affected vanillin degradation; vanillin can be completely converted in 5 h at 160 °C. At 160 °C, the main products of vanillin oxidation were small molecule acids and alcohols, other monophenols, and even condensed dimers. A possible vanillin degradation pathway was proposed. The results indicate that vanillin degradation and condensation are the main reasons for decreasing vanillin yield during lignin valorization under alkaline oxidation circumstances.





Similar content being viewed by others
References
Van den Bosch S, Schutyser W, Vanholme R, Driessen T, Koelewijn SF, Renders T, De Meester B, Huijgen WJJ, Dehaen W, Courtin CM, Lagrain B, Boerjan W, Sels BF (2015) Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ Sci 8(6):1748–1763. https://doi.org/10.1039/c5ee00204d
Renders T, Van den Bosch S, Koelewijn S-F, Schutyser W, Sels BF (2017) Lignin-first biomass fractionation, the advent of active stabilisation strategies. Energy Environ Sci 10(7):1551–1557. https://doi.org/10.1039/C7EE01298E
Song Q, Wang F, Cai JY, Wang YH, Zhang JJ, Yu WQ, Xu J (2013) Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ Sci 6(3):994–1028. https://doi.org/10.1039/c2ee23741e
Deng HB, Lin L, Sun Y, Pang CS, Zhuang JP, Ouyang PK, Li JJ, Liu SJ (2009) Activity and stability of perovskite-type oxide LaCoO3 catalyst in lignin catalytic wet oxidation to aromatic aldehydes process. Energy Fuels 23:19–24. https://doi.org/10.1021/ef8005349
Zakzeski J, Dębczak A, Bruijnincx PCA, Weckhuysen BM (2011) Catalytic oxidation of aromatic oxygenates by the heterogeneous catalyst Co-ZIF-9. Appl Catal A 394(1–2):79–85. https://doi.org/10.1016/j.apcata.2010.12.026
Sales FG, Abreu CAM, Pereira JAFR (2004) Catalytic wet-air oxidation of lignin in a three-phase reactor with aromatic aldehyde production. Braz J Chem Eng 21:211–218. https://doi.org/10.1590/S0104-66322004000200010
Werhan H, Assmann N, von Rohr PR (2013) Lignin oxidation studies in a continuous two-phase flow microreactor. Chem Eng Process 73:29–37. https://doi.org/10.1016/j.cep.2013.06.015
Galkin MV, Smit AT, Subbotina E, Artemenko KA, Bergquist J, Huijgen WJ, Samec JS (2016) Hydrogen-free catalytic fractionation of woody biomass. Chemsuschem 9(23):3280–3287. https://doi.org/10.1002/cssc.201600648
Zakzeski J, Jongerius AL, Weckhuysen BM (2010) Transition metal catalyzed oxidation of Alcell lignin, soda lignin, and lignin model compounds in ionic liquids. Green Chem 12(7):1225–1236. https://doi.org/10.1039/c001389g
Lv W, Si Z, Tian Z, Wang C, Zhang Q, Xu Y, Wang T, Ma L (2017) Synergistic effect of EtOAc/H2O biphasic solvent and Ru/C catalyst for cornstalk hydrolysis residue depolymerization. Acs Sustain Chem Eng 5(4):2981–2993. https://doi.org/10.1021/acssuschemeng.6b02535
Zhang XH, Zhang Q, Wang TJ, Ma LL, Yu YX, Chen LG (2013) Hydrodeoxygenation of lignin-derived phenolic compounds to hydrocarbons over Ni/SiO2–ZrO2 catalysts. Bioresour Technol 134:73–80. https://doi.org/10.1016/j.biortech.2013.02.039
Liu Y, Chen LG, Wang TJ, Zhang Q, Wang CG, Yan JY, Ma LL (2015) One-pot catalytic conversion of raw lignocellulosic biomass into gasoline alkanes and chemicals over LiTaMoO6 and Ru/C in aqueous phosphoric acid. Acs Sustain Chem Eng 3(8):1745–1755. https://doi.org/10.1021/acssuschemeng.5b00256
Li C, Zhao X, Wang A, Huber GW, Zhang T (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev 115(21):11559–11624. https://doi.org/10.1021/acs.chemrev.5b00155
Lange H, Decina S, Crestini C (2013) Oxidative upgrade of lignin—recent routes reviewed. Eur Polym J 49(6):1151–1173. https://doi.org/10.1016/j.eurpolymj.2013.03.002
Fache M, Boutevin B, Caillol S (2016) Vanillin production from lignin and its use as a renewable chemical. Acs Sustain Chem Eng 4(1):35–46. https://doi.org/10.1021/acssuschemeng.5b01344
Rautiainen S, Chen J, Vehkamäki M, Repo T (2016) Oxidation of vanillin with supported gold nanoparticles. Top Catal 59(13–14):1138–1142. https://doi.org/10.1007/s11244-016-0633-8
Patil DG, Magdum PA, Nandibewoor ST (2015) Mechanistic investigations of uncatalyzed and ruthenium(III) catalyzed oxidation of vanillin by periodate in aqueous alkaline medium. J Solution Chem 44(6):1205–1223. https://doi.org/10.1007/s10953-015-0341-1
Fache M, Boutevin B, Caillol S (2015) Vanillin, a key-intermediate of biobased polymers. Eur Polym J 68(SI):488–502. https://doi.org/10.1016/j.eurpolymj.2015.03.050
Bomgardner MM (2014) Following many routes to naturally derived vanillin. Chem Eng News 92(6):14–15. https://doi.org/10.1021/cen-09232-bus2
Tarabanko VE, Koropatchinskaya NV, Kudryashev AV, Kuznetsov BN (1995) Influence of lignin origin on the efficiency of the catalytic oxidation of lignin into vanillin and syringaldehyde. Russ Chem Bull 44:367–371. https://doi.org/10.1007/bf00702154
Tomlinson GH, Hibbert H (1936) Studies on lignin and related compounds. XXV. Mechanism of vanillin formation from spruce lignin sulfonic acids in relation to lignin structure. J Am Chem Soc 58:348–353. https://doi.org/10.1021/ja01293a047
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48. https://doi.org/10.1016/j.biortech.2015.08.085
Behling R, Valange S, Chatel G (2016) Heterogeneous catalytic oxidation for lignin valorization into valuable chemicals: what results? What limitations? What trends? Green Chem 18(7):1839–1854. https://doi.org/10.1039/c5gc03061g
Deng WP, Zhang HX, Wu XJ, Li RS, Zhang QH, Wang Y (2015) Oxidative conversion of lignin and lignin model compounds catalyzed by CeO2-supported Pd nanoparticles. Green Chem 17(11):5009–5018. https://doi.org/10.1039/C5GC01473E
Rodrigues Pinto PC, Borges da Silva EA, Rodrigues AE (2011) Insights into oxidative conversion of lignin to high-added-value phenolic aldehyde. Ind Eng Chem Res 50:741–748. https://doi.org/10.1021/ie102132a
Wu GX, Heitz M, Chornet E (1994) Improved alkaline oxidation process for the production of aldehydes (vanillin and syringaldehyde) from steam-explosion hardwood lignin. Ind Eng Chern Res 33:718–723. https://doi.org/10.1021/ie00027a034
Fargues C, Mathias Á, Silva J, Rodrígues A (1996) Kinetics of vanillin oxidation. Chem Eng Technol 19(2):127–136. https://doi.org/10.1002/ceat.270190206
Sultanov VS, Wallis AFA (1991) Reactivities of guaiacyl and syringyl lignin model phenols towards oxidation with oxygen-alkali. J Wood Chem Technol 11(3):291–305. https://doi.org/10.1080/02773819108050276
Augugliaro V, Camera-Roda G, Loddo V, Palmisano G, Palmisano L, Parrino F, Puma MA (2012) Synthesis of vanillin in water by TiO2 photocatalysis. Appl Catal B 111–112:555–561. https://doi.org/10.1016/j.apcatb.2011.11.007
Bjørsvik H-R (1999) Fine chemicals from lignosulfonates. 1. Synthesis of vanillin by oxidation of lignosulfonates. Org Process Res Dev 3:330–340. https://doi.org/10.1021/op9900028
Tarabanko VE, Petukhov DV, Selyutin GE (2004) New mechanism for the catalytic oxidation of lignin to vanillin. Kinet Catal 45(4):569–577. https://doi.org/10.1023/B:KICA.0000038087.95130.a5
Tarabanko VE, Fomova NA, Kuznetsov BN, Ivanchenko NM, Kudryashev AV (1995) On the mechanism of vanillin formation in the catalytic oxidation of lignin with oxygen. React Kinet Catal Lett 55:161–170. https://doi.org/10.1007/BF02075847
Mathias AL, Rodrigues AB (1995) Production of vanillin by oxidation of pine kraft lignins with oxygen. Holzforschung 49(3):273–278. https://doi.org/10.1515/hfsg.1995.49.3.273
Borges da Silva EA, Zabkova M, Araújo JD, Cateto CA, Barreiro MF, Belgacem MN, Rodrigues AE (2009) An integrated process to produce vanillin and lignin-based polyurethanes from Kraft lignin. Chem Eng Res Des 87(9):1276–1292. https://doi.org/10.1016/j.cherd.2009.05.008
Araújo JDP, Grande CA, Rodrigues AE (2010) Vanillin production from lignin oxidation in a batch reactor. Chem Eng Res Des 88(8):1024–1032. https://doi.org/10.1016/j.cherd.2010.01.021
Klinke HB, Ahring BK, Schmidt S, Thomsen AB (2002) Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour Technol 82(1):15–26. https://doi.org/10.1016/S0960-8524(01)00152-3
Fargues C, Mathias Á, Rodrigues A (1996) Kinetics of vanillin production from kraft lignin oxidation. Ind Eng Chem Res 35(1):28–36. https://doi.org/10.1021/ie950267k
Shilpy M, Ehsan MA, Ali TH, Abd Hamid SB, Ali ME (2015) Performance of cobalt titanate towards H2O2 based catalytic oxidation of lignin model compound. Rsc Adv 5(97):79644–79653. https://doi.org/10.1039/c5ra14227j
Lahive CW, Deuss PJ, Lancefield CS, Sun Z, Cordes DB, Young CM, Tran F, Slawin AM, de Vries JG, Kamer PC, Westwood NJ, Barta K (2016) Advanced model compounds for understanding acid-catalyzed lignin depolymerization: identification of renewable aromatics and a lignin-derived solvent. J Am Chem Soc 138(28):8900–8911. https://doi.org/10.1021/jacs.6b04144
Jiang ZC, Zhang H, He T, Lv XY, Yi J, Li JM, Hu CW (2016) Understanding the cleavage of inter- and intramolecular linkages in corncob residue for utilization of lignin to produce monophenols. Green Chem 18(14):4109–4115. https://doi.org/10.1039/c6gc00798h
Yang HT, Xie YM, Zheng X, Pu YQ, Huang F, Meng XZ, Wu WB, Ragauskas A, Yao L (2016) Comparative study of lignin characteristics from wheat straw obtained by soda-AQ and kraft pretreatment and effect on the following enzymatic hydrolysis process. Bioresour Technol 207:361–369. https://doi.org/10.1016/j.biortech.2016.01.123
Froass PM, Ragauskas AJ, J-e Jiang (1996) Chemical structure of residual lignin from kraft pulp. J Wood Chem Technol 16(4):347–365. https://doi.org/10.1080/02773819608545820
Acknowledgements
This work was supported by NSFC (National Natural Science Foundation of China) project (nos. 51476175, 51606205), the National Natural Science Foundation of China (no. 51536009) and Chinese Academy of Sciences “one hundred talented plan” (no. y507y51001).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection “Lignin Chemistry”; edited by Luis Serrano, Rafael Luque, Bert Sels.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, Y., Liu, J., Liao, Y. et al. Degradation of Vanillin During Lignin Valorization Under Alkaline Oxidation. Top Curr Chem (Z) 376, 29 (2018). https://doi.org/10.1007/s41061-018-0208-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-018-0208-1