Abstract
A novel adsorbent is described for magnetic solid-phase extraction (MSPE) of the aflatoxins AFB1 and AFB2 (AFBs). Magnetic agarose microspheres (MAMs) were functionalized with an aptamer to bind the AFBs which then were quantified by HPLC and on-line post-column photochemical derivatization with fluorescence detection. Streptavidin-conjugated MAMs were synthesized first by a highly reproducible strategy. They possess strong magnetism and high surface area. The MAMs were characterized by transmission electron microscopy, scanning electron microscopy, optical microscopy, laser diffraction particle size analyzer, Fourier transform infrared spectrometry, vibrating sample magnetometry and laser scanning confocal microscopy. Then, the AFB-aptamers were immobilized on MAMs through biotin–streptavidin interaction. Finally, the MSPE is performed by suspending the aptamer-modified MAMs in the sample. They are then collected by an external magnetic field and the AFBs are eluted with methanol/buffer (20:80). Several parameters affecting the coupling, capturing and eluting efficiency were optimized. Under the optimized conditions, the method is fast, has good linearity, high selectivity, and sensitivity. The LODs are 25 pg·mL−1 for AFB1 and 10 pg·mL−1 for AFB2. The binding capacity is 350 ± 8 ng·g−1 for AFB1 and 384 ± 8 ng·g−1 for AFB2, and the precision of the assay is <8%. The method was successfully applied to the analysis of AFBs in spiked maize samples.

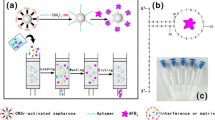
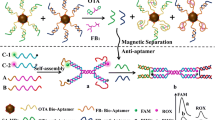
Schematic of novel aptamer functionalized magnetic agarose microspheres (Apt-MAM) as magnetic adsorbents for simultaneous and specific affinity capture of aflatoxins B1 and B2 (AFBs).





Similar content being viewed by others
References
Andrade PD, da Silva JLG, Caldas ED (2013) Simultaneous analysis of aflatoxins B1, B2, G1, G2, M1 and ochratoxin a in breast milk by high-performance liquid chromatography/fluorescence after liquid-liquid extraction with low temperature purification (LLE-LTP). J Chromatogr A 1304:61–68. https://doi.org/10.1016/j.chroma.2013.06.049
Lee KM, Davis J, Herrman TJ, Murray SC, Deng Y (2015) An empirical evaluation of three vibrational spectroscopic methods for detection of aflatoxins in maize. Food Chem 173:629–639. https://doi.org/10.1016/j.foodchem.2014.10.099
Magan N, Medina A, Aldred D (2011) Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol 60:150–163. https://doi.org/10.1111/j.1365-3059.2010.02412.x
Zhang L, Dou XW, Kong WJ, Liu CM, Han X, Yang MH (2017) Assessment of critical points and development of a practical strategy to extend the applicable scope of immunoaffinity column cleanup for aflatoxin detection in medicinal herbs. J Chromatogr A 1483:56–63. https://doi.org/10.1016/j.chroma.2016.12.079
Luan YX, Chen ZB, Xie G, Chen JY, Lu AX, Li C, Fu HL, Ma ZH, Wang JH (2015) Rapid visual detection of aflatoxin B1 by label-free Aptasensor using unmodified gold nanoparticles. J Nanosci Nanotechnol 15:1357–1361. https://doi.org/10.1166/jnn.2015.9225
IARC (International Agency for Research on Cancer). Agents Classified by the IARC Monographs, 2012, Volumes 1–109: Volumes 56, 82, 100F, p 1
Li QF, Lv SZ, Lu MH, Lin ZZ, Tang DP (2016) Potentiometric competitive immunoassay for determination of aflatoxin B1 in food by using antibody-labeled gold nanoparticles. Microchim Acta 183(10):2815–2822. https://doi.org/10.1007/s00604-016-1929-x
Habibipour R, Tamandegani PR, Farmany A (2016) Monitoring of aflatoxin G1, B1, G2, and B2 occurrence in some samples of walnut. Environ Monit Assess 188:669. https://doi.org/10.1007/s10661-016-5678-4
Lai WQ, Zeng Q, Tang J, Zhang MS, Tang DP (2018) A conventional chemical reaction for use in an unconventional assay: A colorimetric immunoassay for aflatoxin B1 by using enzyme-responsive just-in-time generation of a MnO2 based nanocatalyst. Microchim Acta, 185(2), 92. https://doi.org/10.1007/s00604-017-2651-z
Sun LL, Wu LQ, Zhao Q (2017) Aptamer based surface plasmon resonance sensor for aflatoxin B1. Microchim Acta 184(8):2605–2610. https://doi.org/10.1007/s00604-017-2265-5
Lu ZS, Chen XJ, Wang Y, Zheng XT, Li MC (2015) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182(3–4):571–578. https://doi.org/10.1007/s00604-014-1360-0
Wu XM, Hu J, Zhu BH, Lu L, Huang XD, Pang DW (2011) Aptamer-targeted magnetic nanospheres as a solid-phase extraction sorbent for determination of ochratoxin a in food samples. J Chromatogr A 1218:7341–7346. https://doi.org/10.1016/j.chroma.2011.08.045
Synaridou ME, Sakkas VA, Stalikas CD, Albanis TA (2014) Evaluation of magnetic nanoparticles to serve as solid-phase extraction sorbents for the determination of endocrine disruptors in milk samples by gas chromatography mass spectrometry. J Chromatogr A 1348:71–79. https://doi.org/10.1016/j.chroma.2014.04.092
Zhang WM, Yan ZM, Gao J, Tong P, Liu W, Zhang L (2015) Metal-organic framework UiO-66 modified magnetite@silica core-shell magnetic microspheres for magnetic solid-phase extraction of domoic acid from shellfish samples. J Chromatogr A 1400:10–18. https://doi.org/10.1016/j.chroma.2015.04.061
Zhang P, Zhao N, Zeng Z, Feng Y, Tung CH, Chang CC, Zu Y (2009) Using an RNA aptamer probe for flow cytometry detection of CD30-expressing lymphoma cells. Lab Investig 89:1423–1432. https://doi.org/10.1038/labinvest.2009.113
Gopinath SC, Misono TS, Kawasaki K, Mizuno T, Imai M, Odagiri T, Kumar PK (2006) An RNA aptamer that distinguishes between closely related human influenza viruses and inhibits haemagglutinin-mediated membrane fusion. J Gen Virol 87:479–487. https://doi.org/10.1099/vir.0.81508-0
Pavlov V, Shlyahovsky B, Willner I (2005) Fluorescence detection of DNA by the catalytic activation of an aptamer/thrombin complex. J Am Chem Soc 127:6522–6523. https://doi.org/10.1021/ja050678k
Huizeng DE and Szostak JW (1995) A DNA aptamer that binds adenosine and ATP. Biochemistry 34: 656–665. PMID: 7819261
Li BQ, Zhang YC, Huang GH, Cui WR, Zhang N, Cai YD (2014) Prediction of aptamer-target interacting pairs with pseudo-amino acid composition. PLoS One 9:e86729. https://doi.org/10.1371/journal.pone.0086729
Hamaguchi N, Ellington A, Stanton M (2001) Aptamer beacons for the direct detection of proteins. Anal Biochem 294:126–131. https://doi.org/10.1006/abio.2001.5169
Baker BR, Lai RY, Wood MS, Doctor EH, Heeger AJ, Plaxco KW (2006) An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. J Am Chem Soc 128:3138–3139. https://doi.org/10.1021/ja056957p
Wang Y, Yang F, Yang X (2010) Colorimetric biosensing of mercury(II) ion using unmodified gold nanoparticle probes and thrombin-binding aptamer. Biosens Bioelectron 25:1994–1998. https://doi.org/10.1016/j.bios.2010.01.014
Matsumoto I, Mizuno Y, Seno N (1979) Activation of Sepharose with epichlorohydrin and subsequent immobilization of ligand for affinity adsorbent. J Biochem 85: 1091–1098. PMID: 457e633
Liu HM, Luan YX, Lu AX, Li BR, Yang MH, Wang JH (2018) An oligosorbent-based aptamer affinity column for selective extraction of aflatoxin B2 prior to HPLC with fluorometric detection. Microchim Acta 2018(185):71. https://doi.org/10.1007/s00604-017-2591-7
Jiang LF, Chen BC, Chen B, Li XJ, Liao HL, Zhang WY, Wu L (2017) Aptamer-functionalized Fe3O4 magnetic nanoparticles as a solid-phase extraction adsorbent for the selective extraction of berberine from cortex phellodendri. J Sep Sci 40(14):2933–2940. https://doi.org/10.1002/jssc.201700103
Aflatoxins in Corn, Raw peanuts, and peanut butter, in, AOAC Official Method 2005.08, 2006
Yu PF, Wang Q, Zhang XF, Zhang XS, Shen S, Wang Y (2010) Development of superparamagnetic high-magnetization C18-functionalized magnetic silica nanoparticles as sorbents for enrichment and determination of methylprednisolone in rat plasma by high performance liquid chromatography. Anal Chim Acta 678:50–55. https://doi.org/10.1016/j.aca.2010.08.012
Mashhadizadeh MH, Karami Z (2011) Solid phase extraction of trace amounts of ag, cd, cu, and Zn in environmental samples using magnetic nanoparticles coated by 3-(trimethoxysilyl)-1-propantiol and modified with 2-amino-5-mercapto-1,3,4-thiadiazole and their determination by ICP-OES. J Hazard Mater 190:1023–1029. https://doi.org/10.1016/j.jhazmat.2011.04.051
Li JX, Guo ZQ, Zhang SW, Wang XK (2011) Enrich and seal radionuclides in magnetic agarose microspheres. Chem Eng J 172:892–897. https://doi.org/10.1016/j.cej.2011.06.079
European Commission Regulation (EC) No. 401/2006 of laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off J Eur Commun., L70/12 (9.3.2006)
European Commission Regulation (EC) (2010) No 165–2010 amending Regulation (EC) No 1881–2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off J Eur Union L50: 8–12
ISO Guide 33, Reference materials—good practice in using reference materials, 3rd edn., nternational Organization for Standardization, Geneva, 2015
National Verification Regulations and Calibration Specifications of Measuring Instruments of P. R. China, JJF 1507–2015 The effective selection and use of reference materials, General administration of quality supervision, inspection and quarantine of P.R.C
Acknowledgements
The authors are grateful for the support from the Science and Technology project of Beijing (Z171100001317012), Beijing Excellent Talent Training Fund (2017000020060G131), Beijing Academy of Agriculture and Forestry Sciences (KJCX20170401 and KJCX20170419), and the Chinese Postdoctoral Science Foundation funded project (2017 M610807).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interest.
Electronic supplementary material
ESM 1
(DOCX 174 kb)
Rights and permissions
About this article
Cite this article
Liu, H., Lu, A., Fu, H. et al. Affinity capture of aflatoxin B1 and B2 by aptamer-functionalized magnetic agarose microspheres prior to their determination by HPLC. Microchim Acta 185, 326 (2018). https://doi.org/10.1007/s00604-018-2849-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2849-8