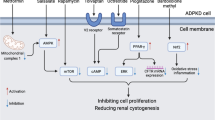
Abstract
One in three Americans is at risk for developing chronic kidney disease (CKD) and end-stage renal disease (ESRD), leading to the need for dialysis or a kidney transplant. Small-molecule drugs have been proposed as therapies to manage kidney diseases, but high dosages are often required to achieve therapeutic efficacy, generating off-target side effects, some of which are lethal. To address these limitations, we developed a novel, kidney-targeting multimodal micelle (KM) system for drug delivery applications. Specifically, we incorporated the kidney-targeting peptide (Lysine-Lysine-Glutamic acid-Glutamic acid-Glutamic acid)3-Lysine) ((KKEEE)3K) into micelles. This peptide binds to megalin, a multi-ligand cell surface receptor present on renal tubule cells. When incubated with human kidney proximal tubule cells, KMs were found to be biocompatible in vitro. In vivo, KMs showed higher accumulation in the kidneys as compared to a non-targeted (NT) control upon intravenous injection in wild-type C57BL/6J mice. Histological evaluation showed no signs of tissue damage, while blood urea nitrogen (BUN) and creatinine levels were within normal ranges, validating the preservation of kidney health upon micelle administration. To our knowledge, this is the first utilization of (KKEEE)3K in a nanoparticle formulation, and our study offers strong evidence that this novel nanoparticle platform can be used as a candidate drug delivery carrier to direct therapeutics to diseased tissue in CKD.
Similar content being viewed by others
References
United States Renal Data System Coordinating Center. 2015 USRDS annual data report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2015.
Wischnjow, A.; Sarko, D.; Janzer, M.; Kaufman, C.; Beijer, B.; Brings, S.; Haberkorn, U.; Larbig, G.; Kübelbeck, A.; Mier, W. Renal targeting: Peptide-based drug delivery to proximal tubule cells. Bioconjug. Chem. 2016, 27, 1050–1057.
Arab, J. P.; Barrera, F.; Arrese, M. Bile acids and portal hypertension. Ann. Hepatol. 2017, 16, s83–s86.
Shimkets, R. A.; Warnock, D. G.; Bositis, C. M.; NelsonWilliams, C.; Hansson, J. H.; Schambelan, M.; Gill, J. R.; Ulick, S.; Milora, R. V.; Findling, J. W. et al. Liddle’s syndrome: Heritable human hypertension caused by mutations in the β subunit of the epithelial sodium channel. Cell 1994, 79, 407–414.
Weixel, K. M.; Marciszyn, A.; Alzamora, R.; Li, H.; Fischer, O.; Edinger, R. S.; Hallows, K. R.; Johnson, J. P. Resveratrol inhibits the epithelial sodium channel via phopshoinositides and AMP-activated protein kinase in kidney collecting duct cells. PLoS One 2013, 8, e78019.
Betrosian, A. P.; Agarwal, B.; Douzinas, E. E. Acute renal dysfunction in liver diseases. World J. Gastroenterol. 2007, 13, 5552–5559.
Chapman, A. B.; Devuyst, O.; Eckardt, K.-U.; Gansevoort, R. T.; Harris, T.; Horie, S.; Kasiske, B. L.; Odland, D.; Pei, Y. P.; Perrone, R. D. et al. Autosomal-dominant polycystic kidney disease (ADPKD): Executive summary from a kidney disease: Improving global outcomes (KDIGO) controversies conference. Kidney Int. 2015, 88, 17–27.
Masoumi, A.; Reed-Gitomer, B.; Kelleher, C.; Bekheirnia, M. R.; Schrier, R. W. Developments in the management of autosomal dominant polycystic kidney disease. Ther. Clin. Risk Manag. 2008, 4, 393–407.
Liu, Y. X.; Ma, X. X.; Zheng, J.; Jia, J. Y.; Yan, T. K. Effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on cardiovascular events and residual renal function in dialysis patients: A meta-analysis of randomised controlled trials. BMC Nephrol. 2017, 18, 206.
Ravnskov, U. Glomerular, tubular and interstitial nephritis associated with non-steroidal antiinflammatory drugs. Evidence of a common mechanism. Br. J. Clin. Pharmacol. 1999, 47, 203–210.
Irazabal, M. V.; Torres, V. E. Experimental therapies and ongoing clinical trials to slow down progression of ADPKD. Curr. Hypertens. Rev. 2013, 9, 44–59.
Takiar, V.; Nishio, S.; Seo-Mayer, P.; King, J. D., Jr; Li, H.; Zhang, L.; Karihaloo, A.; Hallows, K. R.; Somlo, S.; Caplan, M. J. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 2462–2467.
McCreight, L. J.; Bailey, C. J.; Pearson, E. R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435.
Graham, G. G.; Punt, J.; Arora, M.; Day, R. O.; Doogue, M. P.; Duong, J.; Furlong, T. J.; Greenfield, J. R.; Greenup, L. C.; Kirkpatrick, C. M. et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98.
Chung, E. J.; Tirrell, M. Recent advances in targeted, selfassembling nanoparticles to address vascular damage due to atherosclerosis. Adv. Healthc. Mater. 2015, 4, 2408–2422.
Cui, H. G.; Webber, M. J.; Stupp, S. I. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Biopolymers 2010, 94, 1–18.
Trent, A.; Marullo, R.; Lin, B.; Black, M.; Tirrell, M. Structural properties of soluble peptide amphiphile micelles. Soft Matter 2011, 7, 9572–9582.
Choi, C. H. J.; Zuckerman, J. E.; Webster, P.; Davis, M. E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl. Acad. Sci. USA 2011, 108, 6656–6661.
Du, B. J.; Jiang, X. Y.; Das, A.; Zhou, Q. H.; Yu, M. X.; Jin, R. C.; Zheng, J. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat. Nanotechnol. 2017, 12, 1096–1102.
Chung, E. J.; Mlinar, L. B.; Sugimoto, M. J.; Nord, K.; Roman, B. B.; Tirrell, M. In vivo biodistribution and clearance of peptide amphiphile micelles. Nanomedicine 2015, 11, 479–487.
Wang, J.; Masehi-Lano, J. J.; Chung, E. J. Peptide and antibody ligands for renal targeting: Nanomedicine strategies for kidney disease. Biomater. Sci. 2017, 5, 1450–1459.
Janzer, M.; Larbig, G.; Kübelbeck, A.; Wischnjow, A.; Haberkorn, U.; Mier, W. Drug conjugation affects pharmacokinetics and specificity of kidney-targeted peptide carriers. Bioconjug. Chem. 2016, 27, 2441–2449.
Hamley, I. W. Amphiphiles. In Introduction to Soft Matter; Hamley, I.W., Eds.; John Wiley & Sons, Ltd.: Chichester, 2007; pp 161–220.
Verroust, P. J.; Christensen, E. I. Megalin and cubilin—The story of two multipurpose receptors unfolds. Nephrol. Dial. Transplant. 2002, 17, 1867–1871.
Yoo, S. P.; Pineda, F.; Barrett, J. C.; Poon, C.; Tirrell, M.; Chung, E. J. Gadolinium-functionalized peptide amphiphile micelles for multimodal imaging of atherosclerotic lesions. ACS Omega 2016, 1, 996–1003.
Chung, E. J.; Cheng, Y.; Morshed, R.; Nord, K.; Han, Y.; Wegscheid, M. L.; Auffinger, B.; Wainwright, D. A.; Lesniak, M. S.; Tirrell, M. V. Fibrin-binding, peptide amphiphile micelles for targeting glioblastoma. Biomaterials 2014, 35, 1249–1256.
Black, M.; Trent, A.; Kostenko, Y.; Lee, J. S.; Olive, C.; Tirrell, M. Self-assembled peptide amphiphile micelles containing a cytotoxic T-cell epitope promote a protective immune response in vivo. Adv. Mater. 2012, 24, 3845–3849.
Zhou, P.; Sun, X.; Zhang, Z. R. Kidney-targeted drug delivery systems. Acta Pharm. Sin. B 2014, 4, 37–42.
Zavaleta, C.; Ho, D.; Chung, E. J. Theranostic nanoparticles for tracking and monitoring disease state. SLAS Technol. 2018, 23, 281–293.
Poon, C.; Chowdhuri, S.; Kuo, C.-H.; Fang, Y.; Alenghat, F. J.; Hyatt, D.; Kani, K.; Gross, M. E.; Chung, E. J. Protein mimetic and anticancer properties of monocyte-targeting peptide amphiphile micelles. ACS Biomater. Sci. Eng. 2017, 3, 3273–3282.
Khodabandehlou, K.; Masehi-Lano, J. J.; Poon, C.; Wang, J.; Chung, E. J. Targeting cell adhesion molecules with nanoparticles using in vivo and flow-based in vitro models of atherosclerosis. Exp. Biol. Med. 2017, 242, 799–812.
Chung, E. J.; Mlinar, L. B.; Nord, K.; Sugimoto, M. J.; Wonder, E.; Alenghat, F. J.; Fang, Y.; Tirrell, M. Monocytetargeting supramolecular micellar assemblies: A molecular diagnostic tool for atherosclerosis. Adv. Healthc. Mater. 2015, 4, 367–376.
Boron, W. F.; Boulpaep, E. L. Medical Physiology, 3rd ed.; Elsevier: Phildelphia, PA, USA, 2016.
Longmire, M.; Choyke, P. L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717.
Cheng, Y.; Liu, M.; Hu, H. J.; Liu, D. Z.; Zhou, S. Y. Development, optimization, and characterization of PEGylated nanoemulsion of prostaglandin E1 for long circulation. AAPS PharmSciTech 2016, 17, 409–417.
Peters, D.; Kastantin, M.; Kotamraju, V. R.; Karmali, P. P.; Gujraty, K.; Tirrell, M.; Ruoslahti, E. Targeting atherosclerosis by using modular, multifunctional micelles. Proc. Natl. Acad. Sci. USA 2009, 106, 9815–9819.
Chung, E. J.; Pineda, F.; Nord, K.; Karczmar, G.; Lee, S. K.; Tirrell, M. Fibrin-targeting, peptide amphiphile micelles as contrast agents for molecular MRI. J. Cell Sci. Ther. 2014, 5, 181.
Liu, D. M.; Poon, C.; Lu, K. D.; He, C. B.; Lin, W. B. Self-assembled nanoscale coordination polymers with trigger release properties for effective anticancer therapy. Nat. Commun. 2014, 5, 4182.
Yang, L.; Kuang, H. J.; Zhang, W. Y.; Aguilar, Z. P.; Wei, H.; Xu, H. Y. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci. Rep. 2017, 7, 3303.
Chapin, H. C.; Caplan, M. J. The cell biology of polycystic kidney disease. J. Cell Biol. 2010, 191, 701–710.
Slack, A.; Yeoman, A.; Wendon, J. Renal dysfunction in chronic liver disease. Critical Care 2010, 14, 214.
Snanoudj, R.; Durrbach, A.; Gauthier, E.; Adams, D.; Samuel, D.; Ferlicot, S.; Bedossa, P.; Prigent, A.; Bismuth, H.; Charpentier, B. Changes in renal function in patients with familial amyloid polyneuropathy treated with orthotopic liver transplantation. Nephrol. Dial. Transplant. 2004, 19, 1779–1785.
Azmi, A. N.; Tan, S.-S.; Mohamed, R. Hepatitis C and kidney disease: An overview and approach to management. World J. Hepatol. 2015, 7, 78–92.
Gezer, N. S.; Başara, I.; Altay, C.; Harman, M.; Rocher, L.; Karabulut, N.; Seçil, M. Abdominal sarcoidosis: Crosssectional imaging findings. Diagn. Interv. Radiol. 2015, 21, 111–117.
Ng, C. K. F.; Chan, M. H. M.; Tai, M. H. L.; Lam, C. W. K. Hepatorenal syndrome. Clin. Biochem. Rev. 2007, 28, 11–17.
Gonwa, T. A.; Wadei, H. M. Kidney disease in the setting of liver failure: Core curriculum 2013. Am. J. Kidney Dis. 2013, 62, 1198–1212.
Fevery, J.; Van Cutsem, E.; Nevens, F.; Van Steenbergen, W.; Verberckmoes, R.; De Groote, J. Reversal of hepatorenal syndrome in four patients by peroral misoprostol (prostaglandin E1 analogue) and albumin administration. J. Hepatol. 1990, 11, 153–158.
De, S.; Kuwahara, S.; Saito, A. The endocytic receptor megalin and its associated proteins in proximal tubule epithelial cells. Membranes 2014, 4, 333–355.
Tojo, A.; Kinugasa, S. Mechanisms of glomerular albumin filtration and tubular reabsorption. Int. J. Nephrol. 2012, 2012, Article ID 481520.
Monsigny, M.; Roche, A.-C.; Sene, C.; Maget-Dana, R.; Delmotte, F. Sugar-lectin interactions: How does wheat-germ agglutinin bind sialoglycoconjugates? Eur. J. Biochem. 1980, 104, 147–153.
Engel, U.; Breborowicz, D.; Bøg-Hansen, T.; Francis, D. Lectin staining of renal tubules in normal kidney. APMIS 1997, 105, 31–34.
Holthöfer, H. Lectin binding sites in kidney. A comparative study of 14 animal species. J. Histochem. Cytochem. 1983, 31, 531–537.
Rodrigues, W. F.; Miguel, C. B.; Napimoga, M. H.; Oliveira, C. J. F.; Lazo-Chica, J. E. Establishing standards for studying renal function in mice through measurements of body size-adjusted creatinine and urea levels. BioMed Res. Int. 2014, 2014, Article ID 872827.
Dunn, S. R.; Qi, Z. H.; Bottinger, E. P.; Breyer, M. D.; Sharma, K. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int. 2004, 65, 1959–1967.
Hamano, Y.; Grunkemeyer, J. A.; Sudhakar, A.; Zeisberg, M.; Cosgrove, D.; Morello, R.; Lee, B.; Sugimoto, H.; Kalluri, R. Determinants of vascular permeability in the kidney glomerulus. J. Biol. Chem. 2002, 277, 31154–31162.
Kobayashi, H.; Watanabe, R.; Choyke, P. L. Improving conventional enhanced permeability and retention (EPR) effects; What is the appropriate target? Theranostics 2014, 4, 81–89.
Menezes, L. F.; Germino, G. G. Murine models of polycystic kidney disease. Drug Discov. Today Dis. Mech. 2013, 10, e153–e158.
Acknowledgements
The authors would like to acknowledge the financial support from the University of Southern California (USC) Provost Fellowship awarded to J. W., the National Heart, Lung, and Blood Institute (NHLBI), R00HL124279 awarded to E. J. C., and the U.S. Dept. of Defense grant W81XWH-15-1-0420 to K. R. H. The authors also would like to thank the Center for Electron Microscopy and Microanalysis (CEMMA) at USC for assistance in TEM imaging.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, J., Poon, C., Chin, D. et al. Design and in vivo characterization of kidney-targeting multimodal micelles for renal drug delivery. Nano Res. 11, 5584–5595 (2018). https://doi.org/10.1007/s12274-018-2100-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-2100-2