Abstract
A rational-designed conductive sorbent, poly(thiophene-3-boronic acid) electrochemically deposited on a carbon fiber bundle, was applied for boronate affinity extraction. The coated carbon fiber bundle packed into a poly(ether ether ketone) tube was then successfully used for online solid-phase microextraction–high-performance liquid chromatography analysis of cis-diol compounds. Three kinds of catecholamines (namely, adrenaline, noradrenaline, and dopamine) were used as the test analytes. Good extraction efficiency (more than 600-fold), low limits of detection (0.2 ng·mL-1 for adrenaline, 0.1 ng·mL-1 for noradrenaline and dopamine), and wide linear ranges were obtained. The method was demonstrated to be efficient for analysis of catecholamines in spiked plasma samples, with good recoveries in the range of 92.5–95.71%. This work exhibited several significant advantages, including ease of use, high specificity, and high extraction efficiency. It has been demonstrated that such an electrochemically synthesized sorbent has great potential for research involving cis-diol compounds.

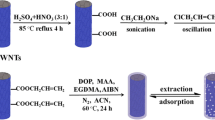
Electrochemical modification, packing of fiber bundles, and extraction mechanism. PEEK poly(ether ether ketone)





Similar content being viewed by others
References
Peaston RT, Weinkove C. Measurement of catecholamines and their metabolites. Ann Clin Biochem. 2004;41(1):17–38.
Kim Y-R, Bong S, Kang Y-J, Yang Y, Mahajan RK, Kim JS, et al. Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens Bioelectron. 2010;25(10):2366–9.
Zan X, Bai H, Wang C, Zhao F, Duan H. Graphene paper decorated with a 2D array of dendritic platinum nanoparticles for ultrasensitive electrochemical detection of dopamine secreted by live cells. Chem Eur J. 2016;22(15):1458-66.
Sajid M, Nazal MK, Mansha M, Alsharaa A, Jillani SMS, Basheer C. Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: a review. Trends Anal Chem. 2016;76:15–29.
Gonon F, Buda M, Cespuglio R, Jouvet M, Pujol J-F. In vivo electrochemical detection of catechols in the neostriatum of anaesthetized rats: dopamine or DOPAC? Nature. 1980;286(28):902–4.
Imperato A, Di Chiara G. Trans-striatal dialysis coupled to reverse phase high performance liquid chromatography with electrochemical detection: a new method for the study of the in vivo release of endogenous dopamine and metabolites. J Neurosci. 1984;4(4):966–77.
Wu D, Xie H, Lu H, Li W, Zhang Q. Sensitive determination of norepinephrine, epinephrine, dopamine and 5-hydroxytryptamine by coupling HPLC with [Ag(HIO6)2]5−-luminol chemiluminescence detection. Biomed Chromatogr. 2016;30(9):1458–66.
Cannazza G, Di Stefano A, Mosciatti B, Braghiroli D, Baraldi M, Pinnen F, et al. Detection of levodopa, dopamine and its metabolites in rat striatum dialysates following peripheral administration of l-DOPA prodrugs by mean of HPLC–EC. J Pharm Biomed Anal. 2005;36(5):1079–84.
Woolley AT, Lao K, Glazer AN, Mathies RA. Capillary electrophoresis chips with integrated electrochemical detection. Anal Chem. 1998;70(4):684–8.
Wallenborg SR, Nyholm L, Lunte CE. End-column amperometric detection in capillary electrophoresis: influence of separation-related parameters on the observed half-wave potential for dopamine and catechol. Anal Chem. 1999;71(3):544–9.
Schöning MJ, Jacobs M, Muck A, Knobbe D-T, Wang J, Chatrathi M, et al. Amperometric PDMS/glass capillary electrophoresis-based biosensor microchip for catechol and dopamine detection. Sens Actuators B. 2005;108(1):688–94.
Thabano JR, Breadmore MC, Hutchinson JP, Johns C, Haddad PR. Capillary electrophoresis of neurotransmitters using in-line solid-phase extraction and preconcentration using a methacrylate-based weak cation-exchange monolithic stationary phase and a pH step gradient. J Chromatogr A. 2007;1175(1):117–26.
Kang QS, Li Y, Xu JQ, Su LJ, Li YT, Huang WH. Polymer monolith-integrated multilayer poly(dimethylsiloxane) microchip for online microextraction and capillary electrophoresis. Electrophoresis. 2010;31(18):3028–34.
Lasáková M, Jandera P. Molecularly imprinted polymers and their application in solid phase extraction. J Sep Sci. 2009;32(5-6):799–812.
Bouri M, Lerma-García MJ, Salghi R, Zougagh M, Ríos A. Selective extraction and determination of catecholamines in urine samples by using a dopamine magnetic molecularly imprinted polymer and capillary electrophoresis. Talanta. 2012;99:897–903.
Nikolajsen RP, Hansen ÅM. Analytical methods for determining urinary catecholamines in healthy subjects. Anal Chim Acta. 2001;449(1):1–15.
Potter OG, Breadmore MC, Hilder EF. Boronate functionalised polymer monoliths for microscale affinity chromatography. Analyst. 2006;131(10):1094–6.
Ren L, Liu Z, Dong M, Ye M, Zou H. Synthesis and characterization of a new boronate affinity monolithic capillary for specific capture of cis-diol-containing compounds. J Chromatogr A. 2009;1216(23):4768–74.
Zhang Q, Tang N, Brock JW, Mottaz HM, Ames JM, Baynes JW, et al. Enrichment and analysis of nonenzymatically glycated peptides: boronate affinity chromatography coupled with electron-transfer dissociation mass spectrometry. J Proteome Res. 2007;6(6):2323–30.
Wang W, He M, Wang C, Wei Y. Enhanced binding capacity of boronate affinity adsorbent via surface modification of silica by combination of atom transfer radical polymerization and chain-end functionalization for high-efficiency enrichment of cis-diol molecules. Anal Chim Acta. 2015;886:66–74.
Li H, Shan Y, Qiao L, Dou A, Shi X, Xu G. Facile synthesis of boronate-decorated polyethyleneimine-grafted hybrid magnetic nanoparticles for the highly selective enrichment of modified nucleosides and ribosylated metabolites. Anal Chem. 2013;85(23):11585–92.
Li D, Li Y, Li X, Bie Z, Pan X, Zhang Q, et al. A high boronate avidity monolithic capillary for the selective enrichment of trace glycoproteins. J Chromatogr A. 2015;1384:88–96.
Wu C, Liang Y, Zhao Q, Qu Y, Zhang S, Wu Q, et al. Boronate affinity monolith with a gold nanoparticle-modified hydrophilic polymer as a matrix for the highly specific capture of glycoproteins. Chem –Eur J. 2014;20(28):8737–43.
Tang J, Liu Y, Qi D, Yao G, Deng C, Zhang X. On-plate-selective enrichment of glycopeptides using boronic acid-modified gold nanoparticles for direct MALDI-QIT-TOF MS analysis. Proteomics. 2009;9(22):5046–55.
Ling X, Zhang W, Chen Z. Electrochemically modified carbon fiber bundles as selective sorbent for online solid-phase microextraction of sulfonamides. Microchim Acta. 2016;183(2):813–20.
Simonet J, Rault-Berthelot J. Electrochemistry: a technique to form, to modify and to characterize organic conducting polymers. Prog Solid State Chem. 1991;21(1):1–48.
Hernandez V, Ramirez F, Otero T, Navarrete JL. An interpretation of the vibrational spectra of insulating and electrically conducting poly (3-methylthiophene) aided by a theoretical dynamical model. J Chem Phys. 1994;100(1):114–29.
Martin DC, Wu J, Shaw CM, King Z, Spanninga SA, Richardson-Burns S, et al. The morphology of poly(3,4-ethylenedioxythiophene). Polym Rev. 2010;50(3):340–84.
Li Y-H, Zhang L, Huang J, Liang R-P, Qiu J-D. Fluorescent graphene quantum dots with a boronic acid appended bipyridinium salt to sense monosaccharides in aqueous solution. Chem Commun. 2013;49(45):5180–2.
Mohapatra S, Panda N, Pramanik P. Boronic acid functionalized superparamagnetic iron oxide nanoparticle as a novel tool for adsorption of sugar. Mater Sci Eng C. 2009;29(7):2254–60.
Kahraman G, Beşkardeş O, Rzaev ZM, Pişkin E. Bioengineering polyfunctional copolymers. VII. Synthesis and characterization of copolymers of p-vinylphenyl boronic acid with maleic and citraconic anhydrides and their self-assembled macrobranched supramolecular architectures. Polymer. 2004;45(17):5813–28.
Lee M, Oh SY, Pathak TS, Paeng IR, Cho B-Y, Paeng K-J. Selective solid-phase extraction of catecholamines by the chemically modified polymeric adsorbents with crown ether. J Chromatogr A. 2007;1160(1–2):340–4.
Raggi M, Sabbioni C, Casamenti G, Gerra G, Calonghi N, Masotti L. Determination of catecholamines in human plasma by high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1999;730(2):201–11.
He J, Liu Z, Ren L, Liu Y, Dou P, Qian K, et al. On-line coupling of in-tube boronate affinity solid phase microextraction with high performance liquid chromatography–electrospray ionization tandem mass spectrometry for the determination of cis-diol biomolecules. Talanta. 2010;82(1):270–6.
Cakal C, Ferrance JP, Landers JP, Caglar P. Microchip extraction of catecholamines using a boronic acid functional affinity monolith. Anal Chim Acta. 2011;690(1):94–100.
Cakal C, Ferrance JP, Landers JP, Caglar P. Development of a micro-total analysis system (μ-TAS) for the determination of catecholamines. Anal Bioanal Chem. 2010;398(5):1909–17.
Acknowledgements
The authors gratefully thank the National Natural Science Foundation of China (grant nos. 21375101, 81573384, and 91417301) and the Natural Science Foundation of Hubei (no. 2014CFA077) for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests. The study received clearance from the Institutional Animal Ethical Committee of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Wuhan University, Wuhan, China.
Electronic supplementary material
ESM 1
(PDF 1.24 MB)
Rights and permissions
About this article
Cite this article
Ling, X., Chen, Z. Boronate affinity solid-phase extraction of cis-diol compounds by a one-step electrochemically synthesized selective polymer sorbent. Anal Bioanal Chem 410, 501–508 (2018). https://doi.org/10.1007/s00216-017-0740-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0740-9